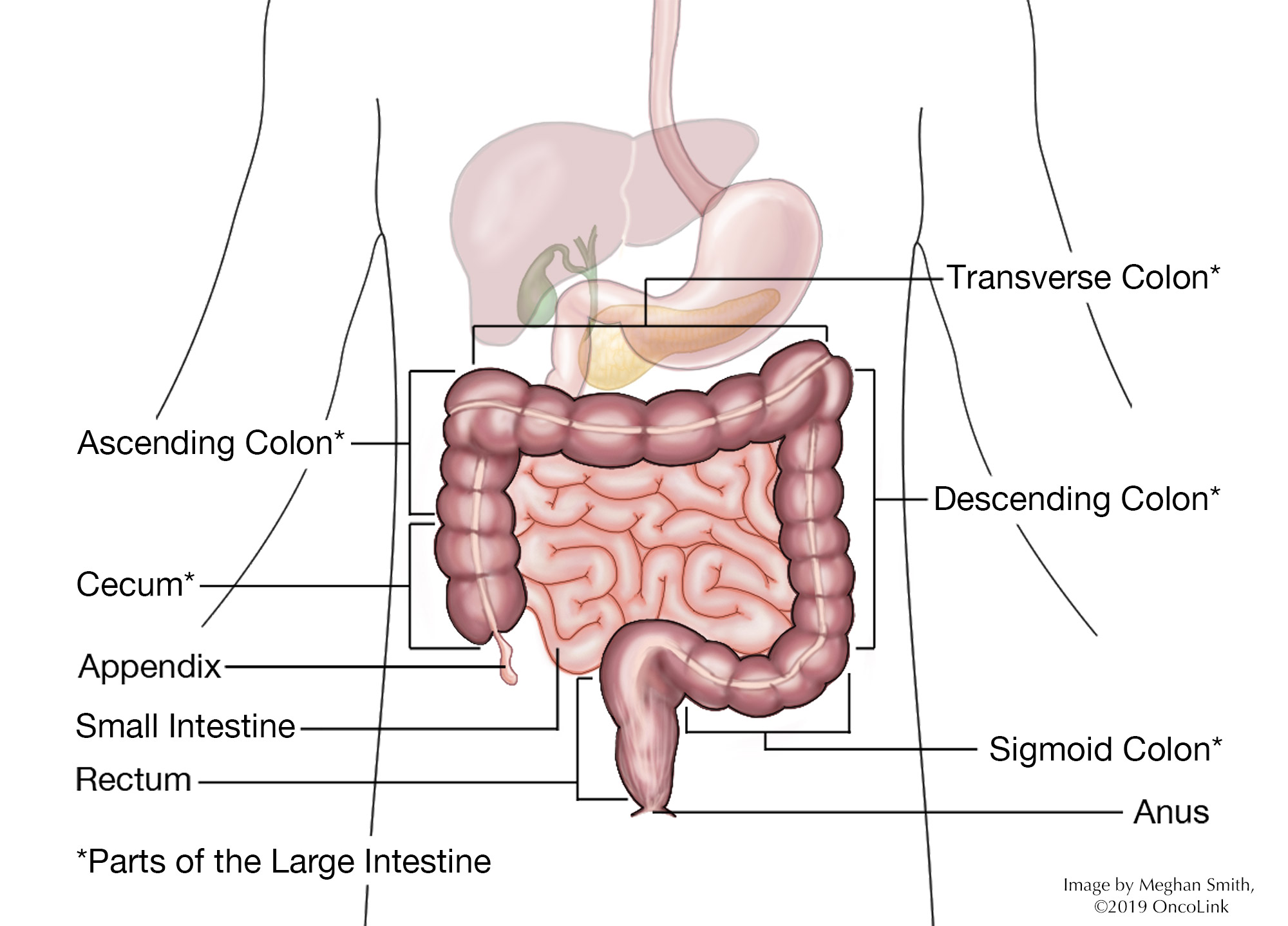
Colon Cancer: Staging and Treatment
What is staging for cancer?
Staging is the process of learning how much cancer is in your body and where it is. For colon cancer, blood tests for tumor marker levels (CEA and CA 19-9), and imaging tests like CT, MRI, and/or PET scans may be used to help
stage your cancer. Your providers need to know about your cancer and your health so that they can plan the best treatment for you.
Staging looks at the size of the tumor and where it is, and if it has spread to other organs. The staging system for colon cancer is called the “TNM system,” as described by the American Joint Committee on Cancer. It has three parts:
- T-describes the size/location/extent of the "primary" tumor in the colon.
- N-describes if the cancer has spread to the lymph nodes.
- M-describes if the cancer has spread to other organs (metastases).
Your healthcare provider will use the results of the tests you had to determine your TNM result and combine these to get a stage from 0 to IV.
How is colon cancer staged?
Staging for colon cancer is based on:
- The location and size of the tumor.
- If the tumor has spread to the lymph nodes. If so, how many lymph nodes are affected.
- If the tumor has spread to other organs. This is called metastasis.
Staging is important because it helps to guide your treatment options. The staging system is very complex. Below is a summary of the staging system. Talk to your provider about the stage of your cancer.
- Stage 0 (Tis, N0, M0): The cancer has not grown beyond the inner layer of the colon. This stage is also called carcinoma in situ or intramucosal carcinoma.
- Stage I (T1 or T2, N0, M0): The cancer has grown through the muscularis mucosa into the submucosa (T1) and may have grown into the muscularis propria (T2). It is not in the lymph nodes or in distant sites.
- Stage IIA (T3, N0, M0): The cancer is in the outermost layers of the colon but has not gone through them. It has not reached nearby organs, lymph nodes, or distant sites.
- Stage IIB (T4a, N0, M0): The cancer has grown through the wall of the colon but not into nearby tissues or organs and has not spread to lymph nodes or to distant sites.
- Stage IIC (T4b, N0, M0): The cancer has grown through the colon wall and is either touching or has grown into nearby tissues or organs. It has not spread to nearby lymph nodes or distant sites.
- Stage IIIA (T1 or T2, N1/N1c, M0): The cancer has grown through the mucosa and into the submucosa and in some cases the muscularis propria. It has spread to 1-3 lymph nodes and into areas of fat near the lymph nodes. It has not spread to distant sites.
- Stage IIIA (T1, N2a, M0): The cancer has grown into the submucosa and has spread to 4-6 nearby lymph nodes. It has not spread to distant sites.
- Stage IIIB (T3 or T4a, N1/N1c, M0): The cancer has grown into the outermost layers of the colon or through the visceral peritoneum but has not reached nearby organs. It has spread to 1-3 nearby lymph nodes or into areas of fat near the lymph nodes. It has not spread to distant sites.
- Stage IIIB (T2 or T3, N2a, M0): The cancer has grown into the muscularis propria or the outermost layers of the colon. It has spread to 4-6 lymph nodes but not to distant sites.
- Stage IIIB (T1 or T2, N2b, M0): The cancer has grown through the mucosa and into the submucosa and in some cases the muscularis propria. It has spread to 7 or more nearby lymph nodes but not to distant sites.
- Stage IIIC (T4a, N2a, M0): The cancer has grown through the wall of the colon but has not reached nearby organs. It has spread to 4-6 nearby lymph nodes but not to distant sites.
- Stage IIIC (T3 or T4a, N2b, M0): The cancer has grown into the outermost layers of the colon or rectum or through the visceral peritoneum but has not reached nearby organs. It has spread to 7 or more nearby lymph nodes but not to distant sites.
- Stage IIIC (T1b, N1/N2, M0): The cancer has grown through the wall of the colon and is attached to or has grown into other nearby tissues or organs. It has spread to at least one nearby lymph node or into areas of fat near the nodes. It has not spread to distant sites.
- Stage IVA (Any T, Any N, M1a): The cancer may or may not have grown through the colon wall and nearby lymph nodes. It has spread to 1 distant organ or distant set of lymph nodes, but not to distant parts of the peritoneum.
- Stage IVB (Any T, Any N, M1b): The cancer may or may not have grown through the wall of the colon and nearby lymph nodes. It has spread to more than 1 distant organ or set of lymph nodes but not to distant parts of the peritoneum.
- Stage IVC (Any T, Any N, M1c): The cancer may or may not have grown through the wall of the colon and nearby lymph nodes. It has spread to distant parts of the peritoneum and may or may not have spread to distant organs or lymph nodes.
How is colon cancer treated?
Treatment for colon cancer is based on the size and location of the tumor and if it has spread to the lymph nodes or other organs. There can be more than one type of treatment used to treat colon cancer. Some of the treatments used are:
- Surgery.
- Chemotherapy.
- Targeted Therapy.
- Immunotherapy.
- Radiation.
- Interventional Radiology.
- Clinical Trials.
Surgery
Surgery is the most common treatment for colon cancer. If the cancer is limited to a polyp, you can have a polypectomy (removal of the polyp), or a local excision, where a small amount of surrounding tissue is also removed.
If the tumor is in the bowel wall or nearby tissues, you will need a partial colectomy. This is the removal of the cancer and part of the bowel. Lymph nodes are removed to see if the cancer has spread. The two ends of the colon are connected to each other so that your bowel can work normally. In some cases, the two ends of the colon cannot be reconnected and a colostomy is needed. A colostomy is an opening in the abdominal wall to allow the passage of stool. This may be temporary or permanent. If your whole colon is removed, called a total colectomy, you will need a permanent colostomy.
Chemotherapy
Chemotherapy is the use of anti-cancer medications to treat cancer. It can be used to treat colon cancer or to lessen your chance of recurrence (the cancer coming back). Your tumor may be tested for specific markers called microsatellite instability (MSI-H) and stability (MSS) because these can help decide which chemotherapy is best for you.
Fluorouracil, oxaliplatin, irinotecan, trifluridine/tipiracil, and capecitabine can be used. These medications are used to both treat and prevent a recurrence.
Targeted Therapy
Targeted therapies can be used to treat recurrent or metastatic colon cancer. These therapies target specific changes on a cell that help cancer grow and spread. Your tumor will be tested for these specific targets. Certain therapies are used for each target:
- Epidermal growth factor receptor (EGFR): Panitumumab and cetuximab.
- KRAS Wild Type: Cetuximab and panitumumab.
- Vascular Endothelial Growth Factor (VEGF): Ramucirumab, bevacizumab, and ziv-afilbercept.
- BRAF V600E: Vemurafenib and encorafenib.
- HER2, WRAS, and BRAF: Trastuzumab, pertuzumab, lapatinib, and fam-tratuzumab deruxtecan-nxki.
Immunotherapy
Immunotherapy uses your body’s own immune system to find and kill cancer cells. It is also called biologic therapy. Immunotherapy medications being used to treat colon cancer are ipilimumab, nivolumab, and pembrolizumab.
Radiation Therapy
Radiation therapy is the use of high-energy x-rays to kill cancer cells. Colon cancer is not often treated with radiation therapy. Radiation is a local treatment aimed at a "target." Once surgery has been used to treat cancer, the "target" or high-risk area for disease recurrence is not very easy to define. If cancer has spread to other organs, chemotherapy (rather than radiation therapy) is able to reach distant areas of the spread of tumor cells.
Radiation can be used before or after surgery with chemotherapy to prevent a recurrence. It can also be used during surgery to kill any cells left behind. If cancer has grown into another organ, attached itself to the abdominal wall, or metastasized to other parts of the body, radiation therapy may be a treatment option. The use of internal radiation therapy (brachytherapy) is sometimes used but research continues to learn how and when it should be used.
Interventional Radiology
Interventional radiology (IR) uses imaging tests to see inside your body so your provider can do procedures without traditional surgery. These are sometimes called "minimally invasive.” It can be used to treat colon cancer metastases. Procedures that can be done are: CT directed biopsies, chemoembolization, radiofrequency ablation, and radioembolization, among others. Your provider will talk to you more about these procedures and if they would be helpful to you.
Clinical Trials
You may be offered a clinical trial as part of your treatment plan. To find out more about current clinical trials, visit the OncoLink Clinical Trials Matching Service.
Making Treatment Decisions
Your care team will make sure you are included in choosing your treatment plan. This can be overwhelming as you may be given a few options to choose from. It feels like an emergency, but you can take a few weeks to meet with different providers and think about your options and what is best for you. This is a personal decision. Friends and family can help you talk through the options and the pros and cons of each, but they cannot make the decision for you. You need to be comfortable with your decision – this will help you move on to the next steps. If you ever have any questions or concerns, be sure to call your team.
You can learn more about colon cancer at OncoLink.org.
References
NCCN Clinical Practice Guidelines: Colon Cancer. www.nccn.org
American Cancer Society. Colorectal Cancer.
Barras, D. (2015). BRAF Mutation in Colorectal Cancer: An Update: Supplementary Issue: Biomarkers for Colon Cancer. Biomarkers in cancer, 7, BIC-S25248.
Burt, R. W., Cannon, J. A., David, D. S., Early, D. S., Ford, J. M., Giardiello, F. M., ... & Jasperson, K. (2013). Colorectal cancer screening. Journal of the National Comprehensive Cancer Network, 11(12), 1538-1575.
Dienstmann, R., Salazar, R., & Tabernero, J. (2015). Personalizing colon cancer adjuvant therapy: selecting optimal treatments for individual patients. Journal of Clinical Oncology, 33(16), 1787-1796.
Douillard, J. Y., Oliner, K. S., Siena, S., Tabernero, J., Burkes, R., Barugel, M., ... & Rivera, F. (2013). Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. New England Journal of Medicine, 369(11), 1023-1034.
Edge SB, Byrd DR, Compton CC, et al., eds. (2010). AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer.
Grothey, A., Van Cutsem, E., Sobrero, A., Siena, S., Falcone, A., Ychou, M., ... & Adenis, A. (2013). Regorafenib monotherapy for previously treated metastatic colorectal cancer: an international, multicentre, randomised, placebo-controlled, phase 3 trial. The Lancet, 381(9863), 303-312.
Gustavsson, B., Carlsson, G., Machover, D., Petrelli, N., Roth, A., Schmoll, H. J., ... & Gibson, F. (2015). A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clinical Colorectal Cancer, 14(1), 1-10.
Heiken, J. P. (2015). CT colonography (‘virtual colonoscopy’): Is it ready for colorectal cancer screening? Cancer Imaging, 3(2), 146.
Heinemann, V., Douillard, J. Y., Ducreux, M., & Peeters, M. (2013). Targeted therapy in metastatic colorectal cancer–an example of personalized medicine in action. Cancer Treatment Reviews, 39(6), 592-601.
Imperiale, T. F., Ransohoff, D. F., Itzkowitz, S. H., Levin, T. R., Lavin, P., Lidgard, G. P., ... & Berger, B. M. (2014). Multitarget stool DNA testing for colorectal-cancer screening. New England Journal of Medicine, 370(14), 1287-1297.
Lee, G. H., Malietzis, G., Askari, A., Bernardo, D., Al-Hassi, H. O., & Clark, S. K. (2015). Is right-sided colon cancer different to left-sided colorectal cancer?–a systematic review. European Journal of Surgical Oncology (EJSO), 41(3), 300-308.
Loupakis, F., Cremolini, C., Masi, G., Lonardi, S., Zagonel, V., Salvatore, L., ... & Zaniboni, A. (2014). Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. New England Journal of Medicine, 371(17), 1609-1618.
Nordlinger, B., Sorbye, H., Glimelius, B., Poston, G. J., Schlag, P. M., Rougier, P., ... & Jaeck, D. (2013). Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. The Lancet Oncology, 14(12), 1208-1215.
Proverbs-Singh, T. A., Marshall, J., Varda, M. M., Nakhoul, I., Balar, B., Lee, J. S., ... & Goldberg, S. L. (2018). Profiling for microsatellite instability (MSI) and mismatch repair (MMR) among patients with colon cancer in real world settings. Journal of Clinical Oncology, 36(15)suppl, e15622-e15622
Regenbogen, S. E., & Hardiman, K. M. (2016). Colorectal Cancer: Surveillance After Curative-Intent Therapy. In The ASCRS Textbook of Colon and Rectal Surgery (pp. 555-570). Springer International Publishing.
Rex, D. K., Boland, C. R., Dominitz, J. A., Giardiello, F. M., Johnson, D. A., Kaltenbach, T., ... & Robertson, D. J. (2017). Colorectal cancer screening: recommendations for physicians and patients from the US Multi-Society Task Force on Colorectal Cancer. The American journal of gastroenterology, 112(7), 1016.
Rodriguez, J., Vicent, S., Chopitea, A., & Ponz-Sarvise, M. (2018). Adjuvant therapy for colon cancer: genes, genes... and the patient in the center. Clinical Cancer Research, clincanres-0818.
Stoffel, E. M., Mangu, P. B., Gruber, S. B., Hamilton, S. R., Kalady, M. F., Lau, M. W. Y., ... & Limburg, P. J. (2014). Hereditary colorectal cancer syndromes: American society of clinical oncology clinical practice guideline endorsement of the familial risk–colorectal cancer: European society for medical oncology clinical practice guidelines. Journal of Clinical Oncology, 33(2), 209-217.
You, Y. N., Rustin, R. B., & Sullivan, J. D. (2015). Oncotype DX® colon cancer assay for prediction of recurrence risk in patients with stage II and III colon cancer: A review of the evidence. Surgical oncology, 24(2), 61-66.