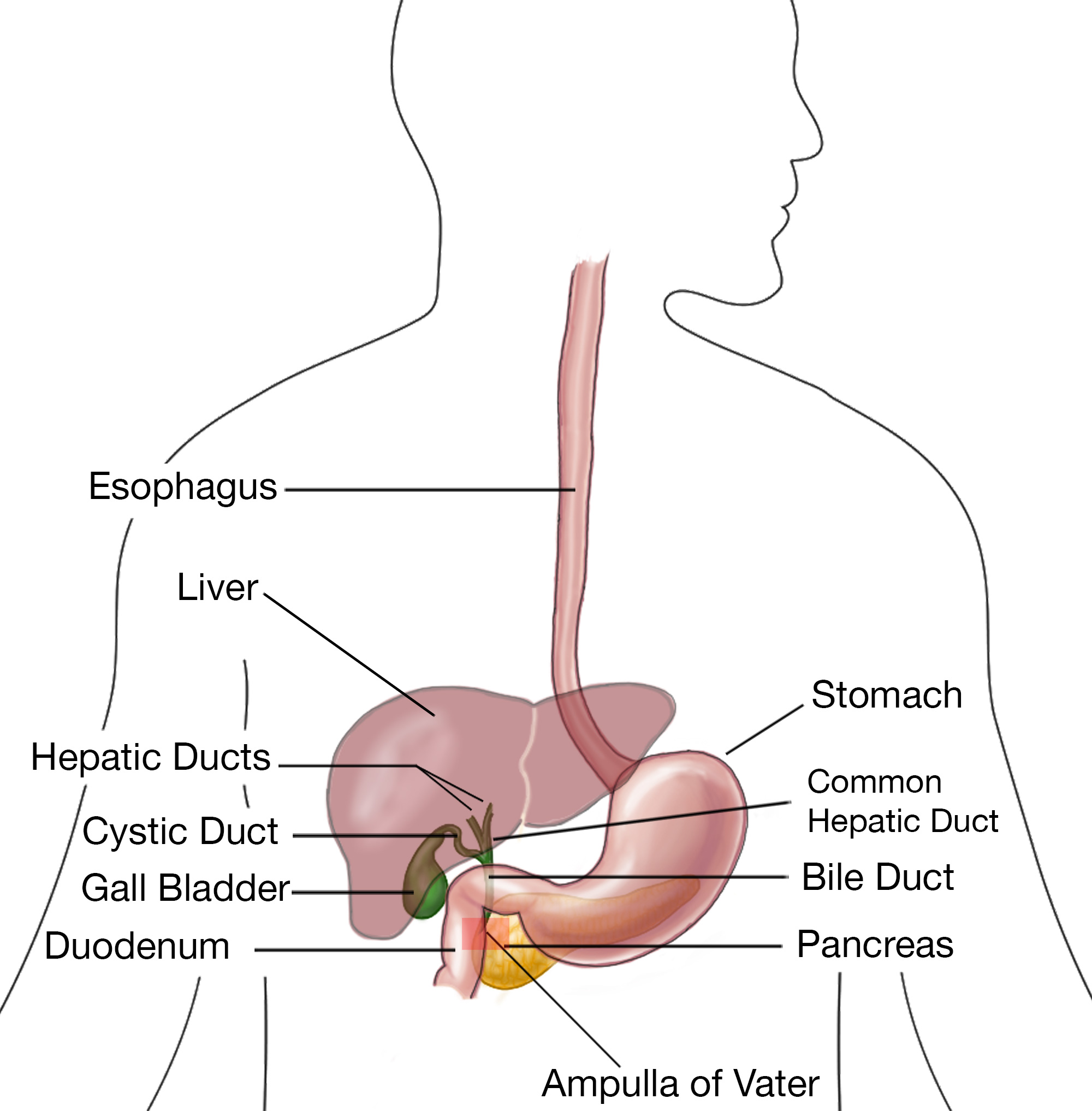
Cholangiocarcinoma (Bile Duct Cancer): Staging and Treatment
What is staging for cancer?
Staging is the process of learning how much cancer is in your body and where it is. Tests like biopsy, ultrasound, CT, MRI, and PET scan may be done to help stage your cancer. Your providers need to know about your cancer and your health so that they can plan the best treatment for you.
Staging looks at the size of the tumor, where it is, and if it has spread to other organs. The staging system for cholangiocarcinoma is called the “TNM system.” The staging has three parts:
- T-describes the size/location/extent of the "primary" tumor in the bile duct.
- N-describes if the cancer has spread to the lymph nodes.
- M-describes if the cancer has spread to other organs (called metastases).
How is cholangiocarcinoma staged?
Staging for bile duct cancer is based on:
- The size of your tumor seen on imaging tests and what is found after surgery.
- Surgery to test if your lymph nodes have cancer cells.
- Any evidence of spread to other organs (metastasis).
There are 3 different staging systems for bile duct cancers, depending on where they start:
- Intrahepatic bile duct cancers: The cancer started in the liver.
- Perihilar (hilar) bile duct cancers: The cancer started in the hilum, the area just outside the liver.
- Distal bile duct cancers: The cancer started farther down the bile duct system.
The staging systems are very complex. Below is a summary. Talk to your provider about the stage of your cancer.
Intrahepatic Bile Duct Cancer Staging
Stage 0 (Tis, N0, M0): The cancer is only in the mucosa (the inner layer of cells in the bile duct). It hasn't started growing into the deeper layers (Tis). It has not spread to nearby lymph nodes (N0) or to distant sites (M0).
Stage IA (T1a, N0, M0): The tumor is no more than 5 cm (about 2 inches) across and has not invaded nearby blood vessels (T1a). It has not spread to nearby lymph nodes (N0) or to distant sites (M0).
Stage IB (T1b, N0, M0): The tumor is more than 5 cm (about 2 inches) across but has not invaded nearby blood vessels (T1b). The cancer has not spread to nearby lymph nodes (N0) or to distant sites (M0).
Stage II (T2, N0, M0): The tumor has grown into nearby blood vessels, OR there are 2 or more tumors, which may or may not have grown into nearby blood vessels (T2). The cancer has not spread to nearby lymph nodes (N0) or to distant sites (M0).
Stage IIIA (T3, N0, M0): The cancer has grown through the visceral peritoneum (the outer lining of organs in the abdomen) (T3). The cancer has not spread to nearby lymph nodes (N0) or to distant sites (M0).
Stage IIIB (T4, N0, M0): The cancer has grown directly into nearby structures outside of the liver (T4). The cancer has not spread to nearby lymph nodes (N0) or to distant sites (M0), OR (Any T, N1, M0): The cancer is any size and might or might not be growing outside the bile duct (Any T) and has spread to nearby lymph nodes (N1). It has not spread to distant sites (M0).
Stage IV (Any T, Any N, M1): The cancer is any size and may or may not be growing outside the bile duct (Any T). It may or may not have spread to nearby lymph nodes (Any N). It has spread to distant organs such as the bones or lungs (M1).
Perihilar (Hilar) Bile Duct Cancer Staging
Stage 0 (Tis, N0, M0): The cancer is only in the mucosa (the innermost layer of cells in the bile duct). It hasn't started growing into the deeper layers (Tis). It has not spread to nearby lymph nodes (N0) or to distant sites (M0).
Stage I (T1, N0, M0): The cancer has grown into deeper layers of the bile duct wall, such as the muscle layer or fibrous tissue layer (T1). It has not spread to nearby lymph nodes (N0) or to distant sites (M0).
Stage II (T2a or T2b, N0, M0): The tumor has grown through the bile duct wall and into the nearby fatty tissue (T2a) or into the nearby liver tissue (T2b). The cancer has not spread to nearby lymph nodes (N0) or to distant sites (M0).
Stage IIIA (T3, N0, M0): The cancer is growing into branches of the main blood vessels of the liver (the portal vein and/or the hepatic artery) on one side (left or right) (T3). The cancer has not spread to nearby lymph nodes (N0) or to distant sites (M0).
Stage IIIB (T4, N0, M0): The cancer is growing into the main blood vessels of the liver (the portal vein and/or the common hepatic artery) or into branches of these vessels on both sides (left and right), OR the cancer is growing into other bile ducts on one side (left or right) and a main blood vessel on the other side (T4). The cancer has not spread to nearby lymph nodes (N0) or to distant sites (M0).
Stage IIIC (Any T, N1, M0): The cancer is any size and may or may not be growing outside the bile duct or into nearby blood vessels (Any T) and has spread to 1 to 3 nearby lymph nodes (N1). It has not spread to distant sites (M0).
Stage IVA (Any T, N2, M0): The cancer is any size and may or may not be growing outside the bile duct or into nearby blood vessels (Any T). It has also spread to 4 or more nearby lymph nodes (N2). It has not spread to distant sites (M0).
Stage IVB (Any T, Any N, M1): The cancer is any size and may or may not be growing outside the bile duct or into nearby blood vessels (Any T). It may or may not have spread to nearby lymph nodes (Any N). It has spread to distant organs such as the bones, lungs, or distant parts of the liver (M1).
Distal Bile Duct Cancer Staging
Stage 0 (Tis, N0, M0): The cancer is only in the mucosa (the innermost layer of cells in the bile duct). It hasn't started growing into the deeper layers (Tis). It has not spread to nearby lymph nodes (N0) or to distant sites (M0).
Stage I (T1, N0, M0): The cancer has grown less than 5 mm (about 1/5 of an inch) into the bile duct wall (T1). It has not spread to nearby lymph nodes (N0) or to distant sites (M0).
Stage IIA (T2, N0, M0): The cancer has grown between 5 mm (about 1/5 of an inch) and 12 mm (about ½ inch) into the bile duct wall (T2). It has not spread to nearby lymph nodes (N0) or to distant sites (M0), OR (T1, N1, M0): The cancer has grown less than 5 mm (about 1/5 of an inch) into the bile duct wall (T1) and has spread to 1 to 3 nearby lymph nodes (N1). It has not spread to distant sites (M0).
Stage IIB (T3, N0, M0): The cancer has grown more than 12 mm (about ½ inch) into the bile duct wall (T3). It has not spread to nearby lymph nodes (N0) or to distant sites (M0), OR (T2 or T3, N1, M0): The cancer has grown 5 mm (about 1/5 of an inch) or more into the bile duct wall (T2 or T3) and has spread to 1 to 3 nearby lymph nodes (N1). It has not spread to distant sites (M0).
Stage IIIA (T1, T2, or T3, N2, M0): The cancer has grown to any depth into the bile duct wall (T1, T2, or T3) and to 4 or more nearby lymph nodes (N2). It has not spread to distant sites (M0).
Stage IIIB (T4, Any N, M0): The cancer is growing into nearby blood vessels (the celiac artery or its branches, the superior mesenteric artery, and/or the common hepatic artery) (T4). The cancer may or may not have spread to nearby lymph nodes (Any N). It has not spread to distant sites (M0).
Stage IV (Any T, Any N, M1): The cancer has grown to any depth within the bile duct wall and may or may not be growing into nearby blood vessels (Any T). It may or may not have spread to nearby lymph nodes (any N). It has spread to distant organs such as the liver, lungs, or peritoneum (inner lining of the abdomen [belly]) (M1).
How is cholangiocarcinoma treated?
Treatment for cholangiocarcinoma depends on many things, like your cancer stage, age, overall health, and testing results. Your treatment may include some or all the following:
Surgery
Surgery can be used to treat some cases of cholangiocarcinoma. Cholangiocarcinoma is either resectable (whole tumor can be removed) or unresectable (some tumor is left in the body).
In some cases, surgery is not an option because of your health or where the tumor is. The goal of surgery is to remove as much of the tumor as possible, with “clean surgical margins.” A clean surgical margin is when the edge of the tissue that is removed has no cancer cells in it. This means all of the tumor has been removed.
If the tumor cannot be removed (unresectable), a stent may be placed. A stent is a small tube that is placed into the bile duct to keep it open and working.
Some treatment centers may do a liver transplant in combination with chemotherapy and radiation for the treatment of intrahepatic cholangiocarcinoma.
There are a few different types of surgeries that can be done. Your provider will talk to you about your options and what treatment may be best for you.
Radiation
Radiation treatment is the use of high-energy x-rays to kill cancer cells. It can be given with external beams of radiation or internal radiation called brachytherapy.
External radiation may be given after surgery to remove part or all of the cancer. This can lower the chance of the cancer coming back (recurrence). It is not known if radiation given after the whole tumor has been removed is helpful in preventing recurrence. Side effects of external beam radiation can be skin irritation, nausea, and fatigue.
In brachytherapy, a catheter (flexible tube) is placed in the area where the tumor is or where it was before it was surgically removed. A radioactive source then travels through the catheter and delivers radiation directly to that area.
Brachytherapy can also be used after stent placement (see below). It has been shown to prevent tumor cells from growing into the stent, helping the stent stay open and working for longer. However, there are some possible serious side effects from brachytherapy, such as cholangitis (infection of the bile ducts), stricture formation (scarring of the bile duct leading to blockage), and ulcer formation in the intestine.
Chemotherapy
Chemotherapy is the use of medications that go throughout the whole body to kill cancer cells. They can be used alone or in combination with surgery and radiation. Often chemotherapy is not used to cure the cancer, instead it is a palliative treatment. This means that it is used to manage side effects and improve your quality of life. The different chemotherapies used are 5-FU, gemcitabine, capecitabine, oxaliplatin, albumin-bound paclitaxel, cisplatin, doxorubicin and regorafenib.
Transcatheter arterial chemoembolization (TACE) is a way to deliver chemotherapy to the tumor area while keeping nearby tissues and organs as safe as possible. This procedure is used most often in intrahepatic (inside the liver) cholangiocarcinomas. During TACE, the tumor is targeted in two ways. First, a very high dose of chemotherapy is delivered directly into the tumor. Second, the blood supply to the tumor is cut off, trapping the anti-cancer drugs within the tumor. As a result, the tumor does not get oxygen and other nutrients it needs to continue to grow. This procedure is done by an interventional radiologist.
Targeted Therapy
Targeted therapy medications attack certain genes and proteins found on cancer cells. Your tumor may be tested for certain genetic markers that can be treated with targeted therapy medications such as pemigatinib, infigratinib, futibatinib, and ivosidenib. There are immunotherapy medications currently being studied in clinical trials.
Palliative Treatment
If your bile duct tumor is unresectable, there are a few treatment options that can help ease symptoms you may have. Palliative treatment focuses on how you feel and can be used to treat or prevent complications (like a blockage in your bile duct).
- Stent placement: A small tube is placed within your bile duct to help keep it open so that bile can flow freely.
- Biliary bypass: If the tumor is blocking the bile duct, a bypass can be made around the tumor. Part of the bile duct before the blockage is connected to a part of the duct that is past the blockage. Often, the gallbladder is used to provide some of the bypass. The bypass lets bile flow into the intestines and can help lessen symptoms, like jaundice(yellowing of skin/eyes) or itching.
- Catheter placement: You may need a way for the bile to drain outside of your body. A catheter is flexible tube. One end of the catheter is in the bile duct and the other end is outside the body. The catheter is attached to a bag outside the body that can be emptied when needed.
- Tumor ablation: A long metal probe is placed through a small hole in the skin and into the tumor. A CT scan or ultrasound is used to guide it to the right place. Either cold (cryosurgery) or heat (radiofrequency) are used on the end of the probe to kill cancer cells.
- Photodynamic therapy (PDT): A special light-activated drug is injected into a vein. This drug collects in cancer cells over a few days. After a few days, an endoscope (a long, flexible tube with a light at the end) is passed down the throat and into the bile ducts. A light on the end of the endoscope shines on the tumor, activating the drug and causing the cells to die. PDT may be used with stenting. If you have PDT, you will be very sensitive to light afterward. Talk with your care team about how to stay safe indoors and outdoors.
Clinical Trials
You may be offered a clinical trial as part of your treatment plan. To find out more about current clinical trials, visit the OncoLink Clinical Trials Matching Services.
Making Treatment Decisions
Your care team will make sure you are included in choosing your treatment plan. This can be overwhelming as you may be given a few options to choose from. It feels like an emergency, but you can take a few weeks to meet with different providers and think about your options and what is best for you. This is a personal decision. Friends and family can help you talk through the options and the pros and cons of each, but they cannot make the decision for you. You need to be comfortable with your decision – this will help you move on to the next steps. If you ever have any questions or concerns, be sure to call your team.
You can learn more about cholangiocarcinoma at OncoLink.org.
References
References
American Cancer Society, https://www.cancer.org/cancer/bile-duct-cancer.html
Amini, A., & Gamblin, T.C. (2014). Palliation. Treating patients with inoperable biliary tract and primary liver tumors. Surgical Oncology Clinics of North America 23(2), 383-397.
Baron, T. H. (2014). Endoscopic retrograde cholangiopancreatography for cholangiocarcinoma. Clinics in Liver Disease, 18(4), 891-897.
Blechacz, B. (2017). Cholangiocarcinoma: Current Knowledge and New Developments. Gut and Liver, 11(1), 13-26.
Boehm, L., et.al. (2014). Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. Journal of Surgical Oncology (in press). Early view retrieved from http://onlinelibrary.wiley.com/doi/10.1002/jso.23781/abstract, 29 Sept 2014.
Brown, K.M., et.al. (2014). Intrahepatic cholangiocarcinoma. Surgical Oncology Clinics of North America 23(2), 231-246.
Bruckner, H. W., Hirschfeld, A., & Schwartz, M. (2016). Targeted therapy for resistant cholangiocarcinoma with bevacizumab or cetuximab added to failed cytotoxic drug cores. Anticancer Research, 36(1), 399-402.
Corona-Villalobos, C.P., Pawlik, T.M., & Kamel, I.R. (2014). Imaging of the patient with a biliary tract or primary liver tumor. Surgical Oncology Clinics of North America 23(2), 189-206.
Fitzgerald, T.L., et.al. (2014). The benefits of liver resection for non-colorectal, non-neuroendocrine liver metastases: a systematic review. Langenbeck's Archives of Surgery (in press). Early view retrieved from http://download.springer.com/static/pdf/557/art%253A10.1007%252Fs00423-014-1241-3.pdf?auth66=1412173424_e42bafa063ee3398d03cf7a6930b0ae6&ext=.pdf, 29 Sept 2014.
Gores, G. J. (2015). Liver transplantation for cholangiocarcinoma. Liver Transplantation, 21(S1).
He, C., Mao, Y., Wang, J., Song, Y., Huang, X., Lin, X., & Li, S. (2018). The Predictive Value of Staging Systems and Inflammation Scores for Patients with Combined Hepatocellular Cholangiocarcinoma After Surgical Resection: a Retrospective Study. Journal of Gastrointestinal Surgery, 1-12.
Hong, M., Cheon, Y.K., Lee, E.J., Lee, T.Y., & Shim, C.S. (2014). Long-term outcome of photodynamic therapy with systemic chemotherapy compared to photodynamic therapy alone in patients with advanced hilar cholangiocarcinoma. Gut and Liver, 8(3), 318-323.
Jarnagin W.R, et al. (2001). Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Annals of Surgery 234, 507-19.
Kambakamba, P., & DeOliveira, M.L. (2014). Perihilar cholangiocarcinoma: paradigms of surgical management. American Journal of Surgery 208 (4)563 -570.
Kim, H.M. et. al. (2014). A pilot study of S-1-based concurrent chemoradiotherapy in patients with biliary tract cancer. Cancer Chemotherapy and Pharmacology (in press). Early view retrieved from http://download.springer.com/static/pdf/833/art%253A10.1007%252Fs00280-014-2565-y.pdf?auth66=1412173498_7b4ff02cdede6ac575ca6ec4a8e49141&ext=.pdf, 29 Sept 2014.
Lad, N., & Kooby, D.A. (2014). Distal cholangiocarcinoma. Surgical Oncology Clinics of North America 23(2), 265-287.
Lee, B.S., et.al. (2014). Risk factors for perihilar cholangiocarcinoma: A hospital-based case-control -study. Liver International(in press). Early view retrieved from http://onlinelibrary.wiley.com/doi/10.1111/liv.12618/abstract, 29 Sept 2014.
Lee, T. Y., Cheon, Y. K., & Shim, C. S. (2013). Current Status of Photodynamic Therapy for Bile Duct Cancer. Clinical Endoscopy, 46(1), 38–44. http://doi.org/10.5946/ce.2013.46.1.38
Lu, S., Zhang, N., Jia, X., Zhang, T., Yang, X., Sun, J., ... & Lu, Y. (2018). Serum CA125, CA199, CEA, CA724, and AFP for diagnosis and prognosis prediction in patients with intrahepatic cholangiocarcinoma. Cancer Biology & Medicine, 15(Suppl 1), 8.
Moeini, A., Sia, D., Bardeesy, N., Mazzaferro, V., & Llovet, J. M. (2016). Molecular Pathogenesis and Targeted Therapies for Intrahepatic Cholangiocarcinoma. Clinical Cancer Research, 22(2), 291-300.
National Comprehensive Cancer Institute http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf, retrieved 30 January 2019 (log-in required)
Razumilava, N., & Gores, G. J. (2014). Cholangiocarcinoma. The Lancet, 383(9935), 2168-2179.
Rizvi, S., Khan, S. A., Hallemeier, C. L., Kelley, R. K., & Gores, G. J. (2018). Cholangiocarcinoma—evolving concepts and therapeutic strategies. Nature Reviews Clinical Oncology, 15(2), 95.
Schmeding, M., & Neumann, U. P. (2015). Liver Transplant for Cholangiocarcinoma: A Comeback?. Experimental and Clinical Transplantation: Official Journal of the Middle East Society for Organ Transplantation, 13(4), 301-308.
Shah, K.N., & Clary, B.M. (2014). Endoscopic and percutaneous approaches to the treatment of biliary tract and primary liver tumors: Controversies and advances. Surgical Oncology Clinics of North America 23(2), 207-230.
Shindoh, J., & Vauthey, J.N. (2014). Staging of biliary tract and primary liver tumors. Surgical Oncology Clinics of North America 23(2), 313-322.
Sithithaworn, P., Yongvanit, P., Duenngai, K., Kiatsopit, N., & Pairojkul, C. (2014). Roles of liver fluke infection as risk factor for cholangiocarcinoma. Journal of Hepato-Biliary-Pancreatic Sciences 21(5), 301-308.
Skipworth, J.R.A., Keane, M.G., & Pereira, S.P. (2014). Update on the management of cholangiocarcinoma. Digestive Diseases 32(5), 570-578.
Steel, J.L., et.al. (2014). Health-related quality of life as a prognostic factor in patients with advanced cancer. Cancer (in press).Early view retrieved from http://onlinelibrary.wiley.com/doi/10.1002/cncr.28902/abstract, 29 Sept 2014.
Thanasuwan, S.,et.al. (2014). Suppression of aquaporin, a mediator of water channel control in the carcinogenic liver fluke, Opisthorchis viverrini. Parasites and Vectors 7(1), 224-233.
Thomas, M.B. (2014). Systemic and targeted therapy for biliary tract tumors and primary liver tumors. Surgical Oncology Clinics of North America 23(2), 369-381.
Vaeteewoottacharn, K., Seubwai, W., Bhudhisawasdi, V., Okada, S., & Wongkham, S. (2014). Potential targeted therapy for liver fluke associated cholangiocarcinoma. Journal of Hepato-Biliary-Pancreatic Sciences 21(6), 362-370.
Wagner, A., Wiedmann, M., Tannapfel, A., Mayr, C., Kiesslich, T., Wolkersdörfer, G. W., ... & Witzigmann, H. (2015). Neoadjuvant Down-Sizing of Hilar Cholangiocarcinoma with Photodynamic Therapy—Long-Term Outcome of a Phase II Pilot Study. International journal of molecular sciences, 16(11), 26619-26628.
Weiss, M.J., Cosgrove, D., et.al. (2014). Multimodal treatment strategies for advanced hilar cholangiocarcinoma. Langenbeck's Archives of Surgery 399(6), 679-692.
Wo, J.Y., Dawson, L.A., Zhu, A.X., & Hong, T.S. (2014). An emerging role for radiation therapy in the treatment of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surgical Oncology Clinics of North America 23(2), 353-368.
Zaydfudim, V.M., Rosen, C.B., & Nagorney, D.M. (2014). Hilar cholangiocarcinoma. Surgical Oncology Clinics of North America 23(2), 247-263.