Colorectal Cancer
Introduction
Colorectal cancer is a major public health problem in western countries, with the highest incidence rates in North America, Western Europe, Australia and New Zealand. It is the third most common cancer in both men and women, and the third most common cause of cancer death in both sexes. Colon cancer is 2.5 times more common than rectal cancer, and both have different natural histories and thus separate treatment strategies. About 90-95% of all colorectal cancers are adenocarcinomas, with the remainder comprised of squamous cell, neuroendocrine or undifferentiated carcinomas.
Case History
History of Present Illness
J.K. is a 53-year old male who presents at the local Emergency Department with a one-week history of unresolving lower abdominal pain. He describes the pain as crampy and "gas-like", located primarily on the right but unlike any "stomach pain" he has ever had before. J.K. states that the pain is worse after meals, but is present throughout the day and occasionally awakens him at night. He denies changes in his bowel habits, though he has not had a bowel movement in the past four days, which he attributes to "bad eating". The patient denies taking any laxatives or stool softeners this week, but admits to heavy ibuprofen use for relief of his discomfort. In addition. his sister, who accompanies him today, comments that he has been somewhat light-headed and low in energy lately, which is unlike his usual demeanor.
Past Medical History
The patient has an history of a right ear carcinoma that was resected and treated with adjuvant radiation and chemotherapy at Temple in 1973. The patient is not certain as to the tumor type, but has not had any complications since that time. He also has a history of GI ulcers that he believes are stress-related.
Past Surgical History
Hemorrhoidectomy 11 years ago
Tonsillectomy childhood
Medications and Allergies
Ibuprofen prn
NKDA
Family History
The patient's mother had ovarian cancer and later died of colon cancer, although he is unsure if the colon disease was primary or metastatic in nature. The patient's two older sisters have both been diagnosed with and treated for colon cancer; they are currently alive and in remission . The patient's grandmother died of colon cancer.
Social History
The patient stopped smoking over 25 years ago, but prior to that he had smoked approximately 1 pack daily for 10 years. He does not drink alcohol or use any illicit drugs. He is a widower, has no children and lives alone in an apartment near his family. The patient is a recently retired carpenter and states that he enjoys reading.
Review of Systems
Denies significant weight loss, but does admit to mildly decreased appetite and difficulty sleeping secondary to his abdominal discomfort. Admits to recent tarry black stools. Otherwise as per HPI.
Physical Examination
GENERAL: The patient is a well-developed, well-nourished African-American male who appears his stated age and is in no acute distress.
VITALS: Within normal.
HEENT: Pale conjunctiva, PERRLA, oropharynx clear.
NECK: No JVD, masses or thyromegaly noted.
CHEST: Clear lung fields bilaterally, chest expansion symmetrical but somewhat limited secondary to abdominal pain.
CARDIOVASCULAR: The heart reveals regular rate and rhythm
ABDOMEN: Soft, voluntary guarding with mild tenderness to palpation of lower right quadrant, NABS x 4
RECTAL: Normal prostate, hemoccult positive stool.
LYMPH: He has no palpable cervical, supraclavicular, or inguinal adenopathy.
EXTREMITIES: No edema or digital clubbing noted.
MUSCULOSKELETAL: His back is nontender to palpation.
NEURO: The patient is alert and oriented x3. He has 5/5 muscle strength in upper and lower extremities bilaterally. His sensation is intact to light touch. There is some drainage from a right ear defect and skin graft secondary to a complication from his prior tonsillectomy. His right cranial nerve VII is non functional. The rest of his cranial nerves II-XII are grossly intact. Data
LABS: Moderate anemia with Hg = 9 mg/dL; CBC and chemistry panel otherwise normal.
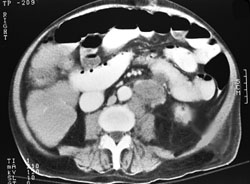
IMAGING: An abdominopelvic CT scan is done which shows enlarged peripancreatic, periaortic, right inferior colonic, small bowel and enteric lymph nodes. The bowel wall appears to be thickened in the right colon and there is obliteration of the lumen of the ascending colon. The examination of the liver reveals no gross lesions but due to poor quality of the scan, the presence of metastasis is not excluded.
Abdominopelvic CT scan slice
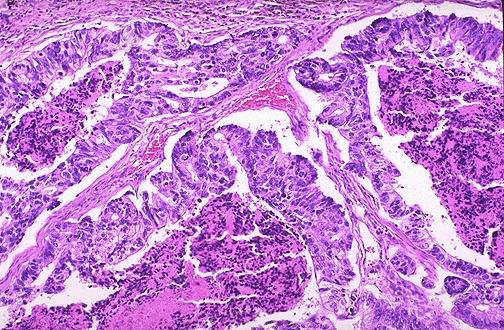
DIAGNOSTIC STUDIES: A colonoscopy is subsequently performed at a later date and shows a right colonic mass. The pathology report from the right colon mass is positive for adenocarcinoma.
Medium-power microscopic view (H & E stain)
Assessment and Plan
The patient is a 53-year old male with a strong family history of colon cancer who presents now with clinical, radiographic and pathologic findings consistent with adenocarcinoma of the colon. He is a strong candidate for surgical intervention for possible therapeutic benefit, as well as vital staging information. The indications, associated risks and alternative options of this recommendation are explained to J.K. and his family. The patient weighs this information and opts to pursue surgery at this time.
Clinical Course
The patient is taken to the operating room for an exploratory laparotomy, but it is determined intraoperatively that the tumor is unresectable and he is subsequently closed without tumor removal. However, an ileotransverse colostomy is performed for relief of an obstructing cecal mass found to be extending into the retroperitoneum.
Postoperatively, J.K. continues to complain of some mild abdominal pain, but is having normal bowel movements. He denies hematochezia or melena, and does not complain of any bony pain or fatigue. There is a periumbilical mass measuring 3x4cm extending to the right side of the umbilicus and firm but mobile upon palpation. He has a midline scar that appears to be well-healed.
The patient's medical oncologist states that this localized, unresectable, T4N3MX adenocarcinoma of the colon may be amenable to treatment using 5-FU chemotherapy combined with localized radiation therapy. The patient is subsequently referred to a radiation oncologist for evaluation. After reviewing J.K.'s case and studies, his radiation oncologist recommends a combined chemoradiotherapy approach. The side effects of radiation and alternative treatments are discussed with the patient and his sister, who both agree to proceed with the chemoradiation.
J.K. tolerates both the infusional 5-FU pump and the radiation therapy well, other than intermittent episodes of nausea and skin hyperpigmentation in the treated area. He continues to eat well and maintain a stable weight. He does not report difficulty with bowel movements. He is currently considering seeing a surgical oncologist to explore the possibility of further surgical intervention.
Discussion
Cancer of the colon is a highly treatable and often curable disease when localized to the bowel. Surgery is the primary form of treatment and results in cure in approximately 50% of patients. Recurrence following surgery is a major problem and often is the ultimate cause of death. The prognosis of colon cancer is clearly related to the degree of penetration of the tumor through the bowel wall and the presence or absence of nodal involvement. These two characteristics form the basis for all staging systems developed for this disease. Bowel obstruction and bowel perforation are indicators of poor prognosis [1]. Elevated pretreatment serum levels of carcinoembryonic antigen (CEA) have a negative prognostic significance [2]. Many other prognostic markers have been evaluated retrospectively in the prognosis of patients with colon cancer, although most, including allelic loss of chromosome 18q or thymidylate synthase expression, have not been prospectively validated [3-5].
Microsatellite instability, not only in association with hereditary nonpolyposis colon cancer, has also been shown to be associated with improved survival independent of tumor stage in a population-based series of 607 patients less than 50 years of age with colorectal cancer [6]. The role of prognostic and predictive markers such as tumor stage, 18q deletion, or microsatellite instability in predicting prognosis or selecting treatment for patients should be determined in prospective trials. Age greater than 65 years at presentation is not a contraindication to standard therapies; acceptable morbidity and mortality, as well as long-term survival, are achieved in this patient population [7-9].
Because of the frequency of the disease, the identification of high-risk groups, the demonstrated slow growth of primary lesions, the better survival of patients with early-stage lesions, and the relative simplicity and accuracy of screening tests, screening for colon cancer should be a part of routine care for all adults starting at age 50 years, especially for those with first-degree relatives with colorectal cancer. There are groups that have a high incidence of colorectal cancer. These groups include those with hereditary conditions, such as familial polyposis, hereditary nonpolyposis colon cancer (HNPCC), Lynch I Syndrome, Lynch II Syndrome, and ulcerative colitis [10]. Together they account for 10% to 15% of colorectal cancers. Patients with HNPCC reportedly have better prognoses in stage-stratified survival analysis than patients with sporadic colorectal cancer, but the retrospective nature of the studies and possibility of selection factors make this observation difficult to interpret [11]. More common conditions with an increased risk include: a personal history of colorectal cancer or adenomas, first degree family history of colorectal cancer or adenomas, and a personal history of ovarian, endometrial, or breast cancer [12,13]. These high-risk groups account for only 23% of all colorectal cancers. Limiting screening or early cancer detection to only these high-risk groups would miss the majority of colorectal cancers [14].
Following treatment of colon cancer, periodic evaluations may lead to the earlier identification and management of recurrent disease [15]. The impact of such monitoring on overall mortality of patients with recurrent colon cancer is limited by the relatively small proportion of patients in whom localized, potentially curable metastases are found. To date, there have been no large-scale randomized trials documenting the efficacy of a standard, postoperative monitoring program [16,17]. Postoperative monitoring may detect asymptomatic, resectable recurrences or metachronous tumors [18-20]. CEA is a serum glycoprotein frequently used in the management of patients with colon cancer. A review of the use of this tumor marker suggests: that CEA is not a valuable screening test for colorectal cancer due to the large numbers of false-positive and false-negative reports; that postoperative CEA testing be restricted to patients who would be candidates for resection of liver or lung metastases; and that routine use of CEA alone for monitoring response to treatment not be recommended [21].
However, the optimal regimen and frequency of follow-up examinations are not well-defined, since the impact on patient survival is not clear and the quality of data is poor [22,23].
Adjuvant therapy
The current generation of large prospective randomized trials has demonstrated consistent evidence of benefit for systemic adjuvant chemotherapy employing fluorouracil (5-FU) plus either levamisole or leucovorin. In 1990, a large intergroup trial of 5-FU/ levamisole reported prolonged disease-free and overall survival in patients with stage III colon cancer, compared to patients who received no treatment after surgery [24]. This benefit has persisted with continued follow-up [25].
The National Surgical Adjuvant Breast and Bowel Project (NSABP) then reported a trial for stage II and III patients comparing the fluorouracil/semustine/vincristine regimen to a weekly regimen of 5-FU plus high-dose leucovorin. This demonstrated a statistically significant benefit for 5-FU/leucovorin in both overall and disease-free survival [26]. Adjuvant 5-FU plus leucovorin (in different treatment schedules) was also compared to surgery alone in 4 large randomized trials that closed prematurely in the early 1990s when surgery alone control arms were no longer felt to represent standard care for stage III patients. Three of these trials, conducted in Canada, France, and Italy, have had their primary data pooled and analyzed together. The 3-year recurrence-free and overall survival rates were also statistically significantly improved in this analysis [27,28]. Taken together, about 4,000 patients have participated in the positive randomized trials comparing adjuvant chemotherapy to surgery alone with a reduction in mortality of between 22% and 33%. These results are quite clear in stage III patients but uncertain in stage II patients. Adjuvant treatment of stage III colon cancer appears to be cost-effective when costs of treatment and quality-of-life measures are taken into account [29].
At this time, patients with stage III (Dukes' C) colon cancer should be considered for adjuvant therapy with 5-FU/leucovorin for 6 to 8 months [30]. Regional adjuvant therapy directed at reducing liver metastasis has been tested using both portal-vein infusion 5-FU and hepatic radiation, and an early trial by Taylor showed promising results [31]. The preliminary results of confirmatory trials from the NSABP, the Mayo Clinic, and the United Kingdom Large Bowel Cancer Project, however, have failed to demonstrate a significant benefit for hepatic-directed adjuvant therapy in the reduction of liver recurrences [32-34]. The NSABP trial, but not the Mayo Clinic trial, showed a modest benefit in survival but no change in the incidence of liver metastases. Neither prolongation of survival nor reduction of liver recurrences was seen in a Gastrointestinal Tumor Study Group study of adjuvant hepatic radiation with 5-FU [35].
Advanced disease
For locally advanced disease, the role of radiation therapy with chemotherapy in colon cancer is under clinical evaluation. Palliation may be achieved in approximately 10% to 20% of patients with 5-FU. Several studies suggest an advantage when leucovorin is added to 5-FU in terms of response rate and palliation of symptoms, but not always in terms of survival [36-42]. Irinotecan (CPT-11) has been approved by the Food and Drug Administration for the treatment of patients whose tumors are refractory to 5-FU [43-46]. Participation in clinical trials is appropriate. A number of other drugs are undergoing early evaluation for the treatment of colon cancer [47]. Oxaliplatin plus 5-FU and leucovorin has also shown activity in 5-FU refractory patients [48,49].
Some retrospective studies suggest that perioperative blood transfusions impair prognosis of patients with colorectal cancer [50,51]. A small, single-institution, prospective randomized trial found the need for allogeneic transfusions following resection of colorectal cancer was an independent predictor of tumor recurrence [52]. This finding was not confirmed by a large, multi-institutional, prospective randomized trial, which actually demonstrated no benefit for autologous blood transfusions when compared to allogeneic transfusions [53]. Both studies established that patients who do not require any blood transfusion have a reduced risk of recurrence, but it would be premature to change transfusion procedures based on these results as other studies have not confirmed this finding [54]. There are a large number of studies correlating various clinical, pathological, and molecular parameters with prognosis, but none of these parameters has been demonstrated to be as important as pathologic stage, and none yet has a major impact on choice of, or outcome from, therapy [3,4,55-61].
References
1. Steinberg SM, Barkin JS, Kaplan RS, et al.: Prognostic indicators of colon tumors: the Gastrointestinal Tumor Study Group experience. Cancer 57(9): 1866-1870, 1986.
2. Filella X, Molina R, Grau JJ, et al.: Prognostic value of CA 19.9 levels in colorectal cancer. Annals of Surgery 216(1): 55-59, 1992.
3. McLeod HL, Murray GI: Tumour markers of prognosis in colorectal cancer. British Journal of Cancer 79(2): 191-203, 1999.
4. Jen J, Kim H, Piantadosi S, et al.: Allelic loss of chromosome 18q and prognosis in colorectal cancer. New England Journal of Medicine 331(4): 213-221, 1994.
5. Lanza G, Matteuzzi M, Gafa R, et al.: Chromosome 18q allelic loss and prognosis in stage II and III colon cancer. International Journal of Cancer 79(4): 390-395, 1998.
6. Gryfe R, Kim H, Hsieh ET, et al.: Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. New England Journal of Medicine 342(2): 69-77, 2000.
7. Fitzgerald SD, Longo WE, Daniel GL, et al.: Advanced colorectal neoplasia in the high-risk elderly patient: is surgical resection justified? Diseases of the Colon and Rectum 36(2): 161-166, 1993.
8. Chiara S, Nobile MT, Vincenti M, et al.: Advanced colorectal cancer in the elderly: results of consecutive trials with 5-fluorouracil-based chemotherapy. Cancer Chemotherapy and Pharmacology 42(4): 336-340, 1998.
9. Popescu RA, Norman A, Ross PJ, et al.: Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. Journal of Clinical Oncology 17(8): 2412-2418, 1999.
10. Thorson AG, Knezetic JA, Lynch HT: A century of progress in hereditary nonpolyposis colorectal cancer (Lynch syndrome). Diseases of the Colon and Rectum 42(1): 1-9, 1999.
11. Watson P, Lin KM, Rodriguez-Bigas MA, et al.: Colorectal carcinoma survival among hereditary nonpolyposis colorectal carcinoma family members. Cancer 83(2): 259-266, 1998.
12. Ransohoff DF, Lang CA: Screening for colorectal cancer. New England Journal of Medicine 325(1): 37-41, 1991.
13. Fuchs CS, Giovannucci EL, Colditz GA, et al.: A prospective study of family history and the risk of colorectal cancer. New England Journal of Medicine 331(25): 1669-1674, 1994.
14. Winawer SJ: Screening for colorectal cancer. Cancer: Principles and Practice of Oncology Updates 2(1): 1-16, 1987.
15. Martin EW, Minton JP, Carey LC: CEA-directed second-look surgery in the asymptomatic patient after primary resection of colorectal carcinoma. Annals of Surgery 202(1): 310-317, 1985.
16. Safi F, Link KH, Beger HG: Is follow-up of colorectal cancer patients worthwhile? Diseases of the Colon and Rectum 36(7):636-644, 1993.
17. Moertel CG, Fleming TR, Macdonald JS, et al.: An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. Journal of the American Medical Association 270(8): 943-947, 1993.
18. Bruinvels DJ, Stiggelbout AM, Kievit J, et al.: Follow-up of patients with colorectal cancer: a meta-analysis. Annals of Surgery 219(2): 174-182, 1994.
19. Lautenbach E, Forde KA, Neugut AI: Benefits of colonoscopic surveillance after curative resection of colorectal cancer. Annals of Surgery 220(2): 206-211, 1994.
20. Khoury DA, Opelka FG, Beck DE, et al.: Colon surveillance after colorectal cancer surgery. Diseases of the Colon and Rectum 39(3):252-256, 1996.
21. American Society of Clinical Oncology: Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Journal of Clinical Oncology 14(10): 2843-2877, 1996.
22. Rosen M, Chan L, Beart RW, et al.: Follow-up of colorectal cancer: a meta-analysis. Diseases of the Colon and Rectum 41(9):1116-1126, 1998.
23. Desch CE, Benson AB III, Smith TJ, et al.: Recommended colorectal cancer surveillance guidelines by the American Society of Clinical Oncology. Journal of Clinical Oncology 17(4): 1312-1321, 1999.
24. Moertel CG, Fleming TR, Macdonald JS, et al.: Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. New England Journal of Medicine 322(6): 352-358, 1990.
25. Moertel CG, Fleming TR, Macdonald JS, et al.: Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Annals of Internal Medicine 122(5):321-326, 1995.
26. Wolmark N, Rockette H, Fisher B, et al.: The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. Journal of Clinical Oncology 11(10): 1879-1887, 1993.
27. International Multicentre Pooled Analysis of Colon Cancer Trials: Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet 345(8955): 939-944, 1995.
28. O'Connell M, Mailliard J, Macdonald J, et al.: An intergroup trial of intensive course 5FU and low dose leucovorin as surgical adjuvant therapy for high risk colon cancer. Proceedings of the American Society of Clinical Oncology 12: A-552, 190, 1993.
29. Brown ML, Nayfield SG, Shibley LM: Adjuvant therapy for stage III colon cancer: economics returns to research and cost-effectiveness of treatment. Journal of the National Cancer Institute 86(6): 424-430, 1994.
30. National Institutes of Health: NIH Consensus Conference: adjuvant therapy for patients with colon and rectal cancer. Journal of the American Medical Association 264(11): 1444-1450, 1990.
31. Taylor I, Machin D, Mullee M, et al.: A randomized controlled trial of adjuvant portal vein cytotoxic perfusion in colorectal cancer. British Journal of Surgery 72(5): 359-363, 1985.
32. Wolmark N, Rockette H, Wickerham DL, et al.: Adjuvant therapy of Dukes' A, B, and C adenocarcinoma of the colon with portal-vein fluorouracil hepatic infusion: preliminary results of National Surgical Adjuvant Breast and Bowel Project Protocol C-02. Journal of Clinical Oncology 8(9): 1466-1475, 1990.
33. Beart RW, Moertel CG, Wieand HS, et al.: Adjuvant therapy for resectable colorectal carcinoma with fluorouracil administered by portal vein infusion: a study of the Mayo Clinic and the North Central Cancer Treatment Group. Archives of Surgery 125(7): 897-901, 1990.
34. Nitti D, Wils J, Sahmoud T, et al.: Final results of a phase III clinical trial on adjuvant intraportal infusion with heparin and 5-fluorouracil (5-FU) in resectable colon cancer (EORTC GITCCG 1983-1987). European Journal of Cancer 33(8): 1209-1215, 1997.
35. The Gastrointestinal Tumor Study Group: Adjuvant therapy with hepatic irradiation plus fluorouracil in colon carcinoma. International Journal of Radiation Oncology, Biology, Physics 21(5): 1151-1156, 1991.
36. Petrelli N, Douglass HO, Herrera L, et al.: The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase III trial. Journal of Clinical Oncology 7(10): 1419-1426, 1989.
37. Erlichman C, Fine S, Wong A, et al.: A randomized trial of fluorouracil and folinic acid in patients with metastatic colorectal carcinoma. Journal of Clinical Oncology 6(3): 469-475, 1988.
38. Doroshow JH, Multhauf P, Leong L, et al.: Prospective randomized comparison of fluorouracil versus fluorouracil and high-dose continuous infusion leucovorin calcium for the treatment of advanced measurable colorectal cancer in patients previously unexposed to chemotherapy. Journal of Clinical Oncology 8(3):491-501, 1990.
39. Poon MA, O'Connell MJ, Wieand HS, et al.: Biochemical modulation of fluorouracil with leucovorin: confirmatory evidence of improved therapeutic efficacy in advanced colorectal cancer. Journal of Clinical Oncology 9(11): 1967-1972, 1991.
40. Valone FH, Friedman MA, Wittlinger PS, et al.: Treatment of patients with advanced colorectal carcinomas with fluorouracil alone, high-dose leucovorin plus fluorouracil, or sequential methotrexate, fluorouracil, and leucovorin: a randomized trial of the Northern
California Oncology Group. Journal of Clinical Oncology 7(10):1427-1436, 1989.
41. Borner MM, Castiglione M, Bacchi M, et al.: The impact of adding low-dose leucovorin to monthly 5-fluorouracil in advanced colorectal carcinoma: results of a phase III trial. Annals of Oncology 9(5):535-541, 1998.
42. Advanced Colorectal Cancer Meta-Analysis Project: Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. Journal of Clinical Oncology 10(6): 896-903, 1992.
43. Rothenberg ML, Eckardt JR, Kuhn JG, et al.: Phase II trial of irinotecan in patients with progressive or rapidly recurrent colorectal cancer. Journal of Clinical Oncology 14(4): 1128-1135, 1996.
44. Conti JA, Kemeny NE, Saltz LB, et al.: Irinotecan is an active agent in untreated patients with metastatic colorectal cancer. Journal of Clinical Oncology 14(3): 709-715, 1996.
45. Rougier P, Van Cutsem E, Bajetta E, et al.: Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 352(9138):1407-1412, 1998.
46. Cunningham D, Pyrhonen S, James RD, et al.: Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 352(9138): 1413-1418, 1998.
47. Von Hoff DD: Promising new agents for treatment of patients with colorectal cancer. Seminars in Oncology 25(5, suppl 11): 47-52,1998.
48. de Gramont A, Vignoud J, Tournigand C, et al.: Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. European Journal of Cancer 33(2): 214-219, 1997.
49. Bleiberg H, de Gramont A: Oxaliplatin plus 5-fluorouracil: clinical experience in patients with advanced colorectal cancer. Seminars in Oncology 25(2 suppl 5): 32-39, 1998.
50. Stephenson KR, Steinberg SM, Hughes KS, et al.: Perioperative blood transfusions are associated with decreased time to recurrence and decreased survival after resection of colorectal liver metastases. Annals of Surgery 208(6): 679-687, 1988.
51. Voogt PJ, Van de Velde CJ, Brand A, et al.: Perioperative blood transfusion and cancer prognosis: different effects of blood transfusion on prognosis of colon and breast cancer patients. Cancer 59(4):836-843, 1987.
52. Heiss MM, Mempel W, Delanoff C, et al.: Blood transfusion-modulated tumor recurrence: first results of a randomized study of autologous versus allogeneic blood transfusion in colorectal cancer surgery. Journal of Clinical Oncology 12(9): 1859-1867, 1994.
53. Busch OR, Hop WC, Hoynck van Papendrecht MA, et al.: Blood transfusions and prognosis in colorectal cancer. New England Journal of Medicine 328(19): 1372-1376, 1993.
54. Donohue JH, Williams S, Cha S, et al.: Perioperative blood transfusions do not affect disease recurrence of patients undergoing curative resection of colorectal carcinoma: a Mayo/North Central Cancer Treatment Group study. Journal of Clinical Oncology 13(7):
1671-1678, 1995.
55. Griffin MR, Bergstralh EJ, Coffey RJ, et al: Predictors of survival after curative resection of carcinoma of the colon and rectum. Cancer 60(9): 2318-2324, 1987.
56. Johnston PG, Fisher ER, Rockette HE, et al.: The role of thymidylate synthase expression in prognosis and outcome of adjuvant chemotherapy in patients with rectal cancer. Journal of Clinical Oncology 12(12): 2640-2647, 1994.
57. Shibata D, Reale MA, Lavin P, et al.: The DCC protein and prognosis in colorectal cancer. New England Journal of Medicine 335(23): 1727-1732, 1996.
58. Bauer KD, Lincoln ST, Vera-Roman JM, et al.: Prognostic implications of proliferative activity and DNA aneuploidy in colonic adenocarcinomas. Laboratory Investigation 57(3): 329-335, 1987.
59. Bauer KD, Bagwell CB, Giaretti W, et al.: Consensus review of the clinical utility of DNA flow cytometry in colorectal cancer. Cytometry 14(5): 486-491, 1993.
60. Sun XF, Carstensen JM, Zhang H, et al.: Prognostic significance of cytoplasmic p53 oncoprotein in colorectal adenocarcinoma. Lancet 340(8832): 1369-1373, 1992.
61. Roth JA: p53 prognostication: pardigm or paradox? Clinical Cancer Research 5(11): 3345, 1999.