National Cancer Institute
Post Date: Mar 5, 2024
Cancer genetics risk assessment and genetic counseling includes family history, psychosocial assessments, and education on hereditary cancer syndromes, testing, and risk. Get more information including the ethical, legal, and social implications of genetic testing in this summary for clinicians.
Cancer Genetics Risk Assessment and Counseling
Executive Summary
This executive summary reviews the topics covered in this PDQ summary on cancer genetics risk assessment and genetic counseling, with hyperlinks to detailed sections below that describe the evidence on each topic.
- Identification of Individuals for Cancer Genetics Risk Assessment and Counseling
Individuals are considered to be candidates for cancer risk assessment if they have a personal and/or family history (on the maternal or paternal side) or clinical characteristics with features suggestive of hereditary cancer. These features vary by type of cancer and specific hereditary syndrome. Criteria have been published to help identify individuals who may benefit from genetic counseling. It is important that individuals who are candidates for genetic testing undergo genetic education and counseling before testing to facilitate informed decision-making and adaptation to the risk or condition. Genetic education and counseling allow individuals to understand the risks, benefits, and limitations of genetic testing. This also allows individuals to consider possible medical uncertainties, cancer diagnoses, and/or medical management plans that accompany certain genetic test results.
- Components of Cancer Genetics Risk Assessment and Counseling
Comprehensive cancer risk assessment and counseling is a consultative service that includes clinical assessment, genetic testing when appropriate, and risk management recommendations delivered in the context of one or more genetic counseling sessions. Pretest genetic counseling is an important part of the risk assessment process and helps patients understand their genetic testing options and potential outcomes. Posttest genetic counseling helps patients understand their test results, including the medical implications for themselves and their relatives.
The recommended provision of cancer risk assessment services optimally involves care providers from multiple disciplines, including a genetic counselor; a genetics advanced practice nurse; a medical geneticist or a physician, such as an oncologist, surgeon, or internist; and potential referrals to other specialists, such as mental health professionals, endocrinologists, and reproductive specialists.
Traditionally, genetic counseling services have been delivered using individualized, in-person appointments. However, other methodologies are being increasingly utilized, including group sessions, telephone counseling, and telemedicine by videoconferencing.
- Genetic Testing Considerations
There are many factors that can influence an individual’s decision to undergo genetic testing and which type of test to use, including the presence of a known pathogenic variant in the family, patterns of cancer in the family, insurance coverage, family planning considerations, and the psychological impact of a test result. Previously, most germline genetic testing was offered for a single gene at a time; however, recent technological advances have resulted in the widespread availability of multigene (panel) testing, which can simultaneously test for pathogenic variants in many genes at once, often at costs comparable to single-gene testing. Research has examined the use and outcomes of multigene testing.
Some health-related cancer genetic tests are also offered as direct-to-consumer (DTC) tests. While these tests may promote access and patient autonomy, the process may not include genetic counseling or interpretation of the results by a genetics professional. In addition, these tests may be incomplete or require confirmation with a second DNA sample sent to another clinical laboratory.
Cascade genetic testing can be effective in identifying carriers of a pathogenic variant prior to cancer presentation which provides opportunities for cancer prevention, early detection, risk reduction, and ultimately improved health outcomes.
Various cancer genetic service delivery approaches are being used to facilitate greater access to genetic counseling and testing. These approaches have been utilized to streamline the process by which high-risk or affected individuals are identified and referred to specialty genetic services for additional evaluation. These service delivery models vary in the processes by which patients receive genetic education, counseling, and testing.
- Ethical, Legal, and Social Implications
Having an understanding of the ethical, legal, and social implications regarding cancer genetic testing may influence the clinician’s response to the complex questions and issues that may arise during the process of risk assessment and counseling. There are several ethical and legal considerations that factor into decisions about what responsibility, if any, providers have to directly inform at-risk relatives about hereditary cancer risks. This section addresses duty to warn, including legal frameworks and available guidance from professional societies. Consultation with an ethicist, ethics committee, legal counsel, privacy officer, and when applicable, an institutional review board, may be warranted in certain disclosure situations.
Employment and insurance discrimination are common concerns for individuals considering genetic testing. The Genetic Information Nondiscrimination Act of 2008 (GINA), a Federal law passed in 2008, protects against health insurance and employment discrimination on the basis of genetics information for most people; however, it does not apply to members of the military or to long-term care, disability, and life insurance provisions.
Introduction
Many of the medical and scientific terms used in this summary are found in the NCI Dictionary of Genetics Terms. When a linked term is clicked, the definition will appear in a separate window.
A concerted effort is being made within the genetics community to shift terminology used to describe genetic variation. The shift is to use the term “variant” rather than the term “mutation” to describe a difference that exists between the person or group being studied and the reference sequence, particularly for differences that exist in the germline. Variants can then be further classified as benign (harmless), likely benign, of uncertain significance, likely pathogenic, or pathogenic (disease causing). Throughout this summary, we will use the term pathogenic variant to describe a disease-causing mutation. For more information about variant classification, see Cancer Genetics Overview.
This summary describes current approaches of assessing and counseling people about their chances of having an inherited susceptibility to cancer. Genetic counseling is defined by the National Society of Genetic Counselors (NSGC) as helping people understand and adapt to the medical, psychological, and familial implications of genetic contributions to disease. Practice resources from NSGC are available to help providers determine hereditary cancer risk and understand guidelines for clinical management of this risk.
Individuals are considered to be candidates for cancer risk assessment if they have personal and/or family histories (in maternal or paternal lineages) with features suggestive of hereditary cancer. These features vary by type of cancer and specific hereditary syndrome. Practice resources have been published to help clinicians identify individuals who may benefit from a genetics evaluation and/or genetic counseling. Organizations like the National Comprehensive Cancer Network frequently update related genetic counseling and genetic testing guidelines. The PDQ cancer genetics information summaries on breast, ovarian, endometrial, colorectal, prostate, kidney, and skin cancers and endocrine and neuroendocrine neoplasias describe the clinical features of hereditary syndromes associated with these conditions.
The following are features that suggest hereditary cancer:
- Unusually early age of cancer onset (e.g., premenopausal breast cancer).
- Multiple primary cancers in a single individual (e.g., colorectal and endometrial cancer).
- Bilateral cancer in paired organs or multifocal disease (e.g., bilateral breast cancer or multifocal renal cancer).
- Clustering of the same type of cancer in close relatives (e.g., mother, daughter, and sisters with breast cancer).
- Cancers occurring in multiple generations of a family (i.e., autosomal dominant inheritance).
- Occurrence of rare tumors (e.g., retinoblastoma, adrenocortical carcinoma, granulosa cell tumor of the ovary, ocular melanoma, or duodenal cancer).
- Occurrence of epithelial ovarian, fallopian tube, or primary peritoneal cancer.
- Unusual presentation of cancer (e.g., male breast cancer).
- Uncommon tumor histology (e.g., medullary thyroid carcinoma).
- Rare cancers associated with birth defects (e.g., Wilms tumor and genitourinary abnormalities).
- Geographic or ethnic populations known to be at high risk of hereditary cancers. Genetic testing candidates may be identified based solely on ethnicity when a strong founder effect is present in a given population (e.g., Ashkenazi heritage and BRCA1/BRCA2pathogenic variants).
As part of the process of genetic education and counseling, genetic testing may be considered when the following factors are present:
- An individual's personal history (including ethnicity) and/or family history are suspicious for a genetic predisposition to cancer.
- The genetic test has sufficient sensitivity and specificity to be interpreted.
- The test will impact the individual's diagnosis, cancer management or cancer risk management, and/or help clarify risk in family members.
It is important that genetic testing candidates undergo genetic education and counseling prior to testing. This process allows greater understanding of disease risk and helps facilitate informed decision making. Genetic education and counseling allow individuals to understand the risks, benefits, and limitations of genetic testing. This also allows individuals to consider possible medical uncertainties, cancer diagnoses, and/or medical management plans that accompany certain genetic test results.
References
- Berliner JL, Cummings SA, Boldt Burnett B, et al.: Risk assessment and genetic counseling for hereditary breast and ovarian cancer syndromes-Practice resource of the National Society of Genetic Counselors. J Genet Couns 30 (2): 342-360, 2021.
- Holter S, Hall MJ, Hampel H, et al.: Risk assessment and genetic counseling for Lynch syndrome - Practice resource of the National Society of Genetic Counselors and the Collaborative Group of the Americas on Inherited Gastrointestinal Cancer. J Genet Couns 31 (3): 568-583, 2022.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 2.2024. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023. Available online with free registration. Last accessed October 31, 2023.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Colorectal. Version 1.2023. Plymouth Meeting, PA: National Comprehensive Cancer Network, 2023. Available with free registration. Last accessed June 28, 2023.
- Hampel H, Bennett RL, Buchanan A, et al.: A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 17 (1): 70-87, 2015.
- Bashford MT, Kohlman W, Everett J, et al.: Addendum: A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 21 (12): 2844, 2019.
- Tobias DH, Eng C, McCurdy LD, et al.: Founder BRCA 1 and 2 mutations among a consecutive series of Ashkenazi Jewish ovarian cancer patients. Gynecol Oncol 78 (2): 148-51, 2000.
- Beller U, Halle D, Catane R, et al.: High frequency of BRCA1 and BRCA2 germline mutations in Ashkenazi Jewish ovarian cancer patients, regardless of family history. Gynecol Oncol 67 (2): 123-6, 1997.
- Gabai-Kapara E, Lahad A, Kaufman B, et al.: Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A 111 (39): 14205-10, 2014.
- Randall LM, Pothuri B, Swisher EM, et al.: Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper. Gynecol Oncol 146 (2): 217-224, 2017.
- Committee on Practice Bulletins–Gynecology, Committee on Genetics, Society of Gynecologic Oncology: Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol 130 (3): e110-e126, 2017.
- Robson ME, Storm CD, Weitzel J, et al.: American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol 28 (5): 893-901, 2010.
- Lancaster JM, Powell CB, Chen LM, et al.: Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol 136 (1): 3-7, 2015.
- Robson ME, Bradbury AR, Arun B, et al.: American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. J Clin Oncol 33 (31): 3660-7, 2015.
Identification of Candidates for Referral to Genetic Counseling
After an individual’s personal and family cancer histories have been collected, several factors could warrant referral to a genetics professional for evaluation of hereditary cancer syndromes. The American College of Medical Genetics and Genomics (ACMG) and the National Society of Genetic Counselors (NSGC) have published a comprehensive set of personal and family history criteria to guide the identification of at-risk individuals and appropriate referral for cancer genetic risk consultation. These practice guidelines address tumor types, other potential features, and related criteria that would prompt a genetics referral. ACMG and NSGC state that the guidelines are intended to maximize appropriate referral of at-risk individuals for cancer genetics consultations, but they are not meant to provide genetic testing or treatment recommendations. Furthermore, ACMG and NSGC acknowledge other sources that provide updated and evolving genetic testing criteria (e.g., the National Comprehensive Cancer Network [NCCN]) and the increasing role of nongenetics professionals in facilitating genetic testing, especially to guide cancer treatment. For more information, see the Cancer Genetics Service Delivery section.
Tools to Identify Candidates for Genetic Counseling and Genetic Testing
All major societies recommend genetic services for patients at a moderate or high risk of having a hereditary cancer syndrome. In addition to published guidelines available through professional organizations, there are also red flag cards, paper-based checklists, chatbots, and patient-directed online referral tools to identify patients who are candidates for genetic counseling and genetic testing. There are a number of commercially available tools (not addressed in this summary) that also offer risk assessment and/or facilitate genetic evaluation. These brief and simple screening tools may be administered to patients in a provider's waiting room or online prior to a visit. Some tools are publicly available and can be accessed directly by patients. For a list of some of these tools, see the table on Risk Assessment Information and the table on Online Pathogenic Variant Prediction Programs in Cancer Genetics Overview.
Many of these tools assess common features suggestive of hereditary cancer, but there are limitations. For example, there is variability in the tools' abilities to flag at-risk individuals based on extensive criteria, as outlined by current professional society guidelines (e.g., NCCN). Hence, some people who are candidates for genetic counseling and genetic testing will be missed by these tools. This includes individuals who may be considered low-risk based on these screening tools. Because of the potential clinical utility of genetic testing, clinical judgement may still be needed to determine the appropriateness of genetic counseling and testing in these individuals. Thus, clinical review of information on screening forms is still warranted.
Additional tools are available to assess the risk of harboring a specific pathogenic variant in a cancer susceptibility gene. For more information, see the Models for Prediction of Breast and Gynecologic Cancer Risk section in Genetics of Breast and Gynecologic Cancers and the Clinical risk assessment models that predict the likelihood of an MMR gene pathogenic variant section in Genetics of Colorectal Cancer.
References
- Hampel H, Bennett RL, Buchanan A, et al.: A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 17 (1): 70-87, 2015.
- Bashford MT, Kohlman W, Everett J, et al.: Addendum: A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 21 (12): 2844, 2019.
- Lancaster JM, Powell CB, Chen LM, et al.: Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol 136 (1): 3-7, 2015.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 2.2024. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023. Available online with free registration. Last accessed October 31, 2023.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Colorectal. Version 1.2023. Plymouth Meeting, PA: National Comprehensive Cancer Network, 2023. Available with free registration. Last accessed June 28, 2023.
Cancer Risk Assessment and Counseling
Comprehensive cancer risk assessment is a consultative service that includes clinical assessment, genetic testing when appropriate, and risk management recommendations delivered in the context of one or more genetic counseling sessions. Pretest genetic counseling is an important part of the risk assessment process and helps patients understand their genetic testing options and potential outcomes. Posttest genetic counseling helps patients understand their test results, including the medical implications for themselves and their relatives.
The following professional organizations emphasize the importance of genetic counseling in the cancer risk assessment and genetic testing process:
- American College of Medical Genetics and Genomics.
- American College of Obstetrics and Gynecology.
- American Society of Clinical Oncology.
- International Society of Nurses in Genetics.
- National Society of Genetic Counselors.
- National Comprehensive Cancer Network.
- Oncology Nursing Society.
- Society of Gynecologic Oncologists.
- U.S. Preventive Services Task Force.
For a list of organizations that have published clinical practices guidelines related to genetic counseling, risk assessment, genetic testing, and/or management for hereditary breast and gynecologic cancers, see the Indications for hereditary breast and gynecologic cancers genetic testing section in Genetics of Breast and Gynecologic Cancers.
Genetic counseling informs the consultand about potential cancer risks and the benefits and limitations of genetic testing and offers an opportunity to consider the potential medical, psychological, familial, and social implications of genetic information. Descriptions of genetic counseling and the specialized practice of cancer risk assessment counseling are detailed below.
Genetic Counseling
Genetic counseling has been defined by the National Society of Genetic Counselors as the process of helping people understand and adapt to the medical, psychological, and familial implications of genetic contributions to disease, including the following:
- How inherited diseases and conditions might affect them or their families.
- How family and medical histories may impact the chance of disease occurrence or recurrence.
- Which genetic tests may or may not be right for them, and what those tests may or may not tell.
- How to make the most informed choices about health care conditions and risk.
Central to the philosophy and practice of genetic counseling are the principles of voluntary utilization of services, informed decision making, attention to psychosocial and affective dimensions of coping with genetic risk, and protection of patient confidentiality and privacy. This is facilitated through a combination of rapport building and information gathering; establishing or verifying diagnoses; risk assessment and calculation of quantitative occurrence/recurrence risks; education and informed consent processes; psychosocial assessment, support, and counseling appropriate to a family’s culture and ethnicity; and other relevant background characteristics. The psychosocial assessment is especially important in the genetic counseling process because individuals most vulnerable to adverse effects of genetic information may include those who have had difficulty dealing with stressful life events in the past. Variables that may influence psychosocial adjustment to genetic information include individual and familial factors; cultural factors; and health system factors such as the type of test, disease status, and risk information. Findings from a psychosocial assessment can be used to help guide the direction of the counseling session. An important objective of genetic counseling is to provide an opportunity for shared decision making when the medical benefits of one course of action are not demonstrated to be superior to another. The relationship between the availability of effective medical treatment for carriers of pathogenic variants and the clinical validity of a given test affects the degree to which personal choice or physician recommendation is supported in counseling at-risk individuals. Uptake of genetic counseling services among those referred varies based on the cancer syndrome and the clinical setting. Efforts to decrease barriers to service utilization are ongoing (e.g., the use of a patient navigator or an oncology clinic–based genetic counselor may increase utilization of these services). Readers interested in the nature and history of genetic counseling are referred to a number of comprehensive reviews.
Pretest Genetic Education and Counseling Outcomes
Cancer risk assessment counseling has emerged as a specialized practice that requires knowledge of genetics, oncology, and individual and family counseling skills that may be provided by health care providers with this interdisciplinary training. Some centers providing cancer risk assessment services involve a multidisciplinary team, which may include a genetic counselor; a genetics advanced practice nurse; a medical geneticist or a physician, such as an oncologist, surgeon, or internist; and a mental health professional.
References
- Hampel H, Bennett RL, Buchanan A, et al.: A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 17 (1): 70-87, 2015.
- Bashford MT, Kohlman W, Everett J, et al.: Addendum: A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 21 (12): 2844, 2019.
- Committee on Practice Bulletins–Gynecology, Committee on Genetics, Society of Gynecologic Oncology: Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol 130 (3): e110-e126, 2017.
- Robson ME, Bradbury AR, Arun B, et al.: American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. J Clin Oncol 33 (31): 3660-7, 2015.
- International Society of Nurses in Genetics: Provision of Quality Genetic Services and Care: Building a Multidisciplinary, Collaborative Approach among Genetic Nurses and Genetic Counselors. Pittsburgh, Pa: International Society of Nurses in Genetics, 2006. Available online. Last accessed January 17, 2024.
- International Society of Nurses in Genetics: Genetic Counseling for Vulnerable Populations: The Role of Nursing. Pittsburgh, Pa: International Society of Nurses in Genetics, 2010. Available online. Last accessed January 17, 2024.
- Resta R, Biesecker BB, Bennett RL, et al.: A new definition of Genetic Counseling: National Society of Genetic Counselors' Task Force report. J Genet Couns 15 (2): 77-83, 2006.
- Berliner JL, Cummings SA, Boldt Burnett B, et al.: Risk assessment and genetic counseling for hereditary breast and ovarian cancer syndromes-Practice resource of the National Society of Genetic Counselors. J Genet Couns 30 (2): 342-360, 2021.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 2.2024. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023. Available online with free registration. Last accessed October 31, 2023.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Colorectal. Version 1.2023. Plymouth Meeting, PA: National Comprehensive Cancer Network, 2023. Available with free registration. Last accessed June 28, 2023.
- Oncology nursing: the application of cancer genetics and genomics throughout the oncology care continuum. Oncol Nurs Forum 40 (1): 10-1, 2013.
- Lancaster JM, Powell CB, Chen LM, et al.: Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol 136 (1): 3-7, 2015.
- Randall LM, Pothuri B, Swisher EM, et al.: Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper. Gynecol Oncol 146 (2): 217-224, 2017.
- Owens DK, Davidson KW, Krist AH, et al.: Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 322 (7): 652-665, 2019.
- Resta RG: Defining and redefining the scope and goals of genetic counseling. Am J Med Genet C Semin Med Genet 142C (4): 269-75, 2006.
- Accreditation Council for Genetic Counseling: Practice-Based Competencies for Genetic Counselors. Accreditation Council for Genetic Counseling, 2023. Available online. Last accessed January 8, 2024.
- Hirschberg AM, Chan-Smutko G, Pirl WF: Psychiatric implications of cancer genetic testing. Cancer 121 (3): 341-60, 2015.
- Riley BD, Culver JO, Skrzynia C, et al.: Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J Genet Couns 21 (2): 151-61, 2012.
- Burke W, Pinsky LE, Press NA: Categorizing genetic tests to identify their ethical, legal, and social implications. Am J Med Genet 106 (3): 233-40, 2001 Fall.
- Rahm AK, Sukhanova A, Ellis J, et al.: Increasing utilization of cancer genetic counseling services using a patient navigator model. J Genet Couns 16 (2): 171-7, 2007.
- Kentwell M, Dow E, Antill Y, et al.: Mainstreaming cancer genetics: A model integrating germline BRCA testing into routine ovarian cancer clinics. Gynecol Oncol 145 (1): 130-136, 2017.
- Kishan AU, Gomez CL, Dawson NA, et al.: Increasing Appropriate BRCA1/2 Mutation Testing: The Role of Family History Documentation and Genetic Counseling in a Multidisciplinary Clinic. Ann Surg Oncol 23 (Suppl 5): 634-641, 2016.
- Walker AP: The practice of genetic counseling. In: Baker DL, Schuette JL, Uhlmann WR, eds.: A Guide to Genetic Counseling. Wiley-Liss, 1998, pp 1-26.
- Bartels DM, LeRoy BS, Caplan AL, eds.: Prescribing Our Future: Ethical Challenges in Genetic Counseling. Aldine De Gruyter, 1993.
- Kenen RH: Genetic counseling: the development of a new interdisciplinary occupational field. Soc Sci Med 18 (7): 541-9, 1984.
- Kenen RH, Smith AC: Genetic counseling for the next 25 years: models for the future. J Genet Couns 4 (2): 115-24, 1995.
- Biesecker BB: Goals of genetic counseling. Clin Genet 60 (5): 323-30, 2001.
- Weil Jon: Psychosocial Genetic Counseling. Oxford University Press, 2000.
- Freedman AN, Wideroff L, Olson L, et al.: US physicians' attitudes toward genetic testing for cancer susceptibility. Am J Med Genet A 120A (1): 63-71, 2003.
Components of the Risk Assessment Process
This section provides an overview of critical elements in the cancer risk assessment process.
A number of professional guidelines on the elements of cancer genetics risk assessment and counseling are available. Except where noted, the discussion below is based on these guidelines.
The cancer risk assessment and genetic counseling process consists of one or more consultative sessions and generally includes the following:
- A detailed, multifaceted assessment including medical, psychosocial, and family history.
- A determination of the risk of cancer and/or indication for genetic testing based on evidence of an inherited cancer syndrome.
- Education and counseling about familial/hereditary cancer risks.
- If appropriate, review of genetic testing options as well as potential limitations, risks, and benefits of testing.
- Establishment of a cancer risk management plan.
- Discussion of follow-up plans, provision of referrals, educational materials, etc.
Assessment
At the outset of the initial counseling session, eliciting and addressing the consultand's perceptions and concerns about cancer and his or her expectations of the risk assessment process helps to engage the consultand in the session. This also helps inform the provider about practical or psychosocial issues and guides the focus of counseling and strategies for risk assessment.
Psychosocial assessment
The genetic counseling process that takes place as part of a cancer risk assessment can identify factors that contribute to the consultand's perception of cancer risk and motivations to seek cancer risk assessment and genetic testing. It can also identify potential psychological issues that may need to be addressed during or after the session, particularly after genetic testing. Information collected before and/or during the session may include the following:
- Motivations for seeking cancer risk assessment.
- Beliefs about the causes of cancer.
- Experiences with cancer and feelings, perceptions, concerns, or fears related to those experiences.
- The influence of cancer experiences and perceptions on health behaviors and cancer screening practices.
- Cultural, religious, and socioeconomic backgrounds.
- General psychological history, such as a history of depression/anxiety, medication use, and ongoing treatments for psychiatric illnesses.
- Coping mechanisms.
- Support systems.
- Cognitive deficits in the consultand, which may limit his/her understanding of the genetic information provided and/or hinder his/her ability to give informed consent.
- Patterns of communication within the family, including cohesion/closeness of family members (or lack thereof), and the family beliefs/values that affect health behaviors. Ethnic and cultural factors may also play an important role in guiding behavior in some families.
- The health of family members (i.e., new diagnoses of cancer or deaths from cancer) and their relationship statuses (i.e., divorced, married, grieving) may inform the provider about the timing of the individual’s participation in genetic counseling or testing. These factors may also reveal possible contraindications for genetic testing at the time of the patient’s genetic counseling session.
Either alone or in consultation with a mental health provider, health care providers offering cancer risk counseling attempt to assess whether there are factors suggesting risk of adverse psychological outcomes after disclosure of risk and/or genetic status.
Risk perception
Perceived risk can play an important role in an individual’s decision to participate in counseling, despite the fact that perceived risk often varies substantially from statistical risk estimates.
Clinical Evaluation
Personal health history
Consideration of the consultand's personal health history is essential in cancer risk assessment, regardless of whether the individual has a personal history of cancer. Important information to obtain about the consultand's health history includes the following:
- Current age.
- Race, ancestry, and ethnicity.
- History of benign or precancerous tumors or polyps, surgeries, biopsies, major illnesses, medications, and reproductive history (for women, this includes age at menarche, parity, age at first live birth, age at menopause, and history of exogenous hormone use).
- Screening practices and date of last screening exams, including imaging and/or physical examinations.
- Environmental exposures.
- Past and current alcohol intake and tobacco use.
- Diet, exercise, and complementary and alternative medicine practices may also be assessed.
For consultands with a history of cancer, additional information collected includes the following:
- Site/type of primary malignancy and any metastasis or recurrence.
- Age at diagnosis.
- Pathology findings/staging.
- Prior germline genetic testing results.
- Prior tumor testing results (including genomic profiling). For more information on the implications of tumor testing, see the Clinical Sequencing section in Cancer Genetics Overview.
- Treatment (e.g., surgery, chemotherapy, radiation therapy, targeted therapy), including whether genetic risk assessment may affect treatment.
- Bilaterality of disease, if applicable.
- Current surveillance plan.
- Carcinogenic exposures (e.g., alcohol and tobacco use, sun exposure, radiation exposure, asbestos exposure) or other known cancer site-specific risk factors.
- How the cancer was detected (e.g., self-exam, screening test, presenting symptoms) may also be assessed.
Physical examination
In some cases, a physical exam is conducted by a qualified medical professional to determine whether the individual has physical findings suggestive of a hereditary cancer predisposition syndrome or to rule out evidence of an existing malignancy. For example, a medical professional may look for the sebaceous adenomas seen in Muir-Torre syndrome, measure the head circumference or perform a skin exam to rule out benign cutaneous features associated with Cowden syndrome, or perform a clinical breast and axillary lymph node exam on a woman undergoing a breast cancer risk assessment.
Family history
Documenting the family history
The family history is an essential tool for cancer risk assessment. Family history may be collected before or during a clinical encounter via an interview with a clinician. Self-administered family history collection tools are an alternative and may improve efficiency. A meta-analysis of 28 studies investigating 27 electronic family history tools reported that patients found these tools acceptable and efficient (average completion time, 31 minutes). This meta-analysis also showed that these tools had high completion rates (average completion rate, 86%) and were more comprehensive when compared with paper-based surveys and clinician interviews. In these studies, family history collection occurred in one of two ways: 1) online before visits, or 2) in clinic via kiosks or electronic tablets. However, it was challenging to integrate tool-collected information into electronic medical records, and functionality was also an issue. Collecting family history from multiple relatives in a single family has been shown to increase the number of family members reported to have cancer, compared with family history information provided by a single family member.
Details of the family health history are best summarized in the form of a family tree, or pedigree. The pedigree, a standardized graphic representation of family relationships, facilitates identification of patterns of disease transmission, recognition of the clinical characteristics associated with specific hereditary cancer syndromes, and determination of the best strategies and tools for risk assessment.
Current standards of pedigree nomenclature have been published by the National Society of Genetic Counselors (NSGC) and adopted by the National Comprehensive Cancer Network. See these referenced works for detailed explanations, discussion, and examples of pedigree symbols.
Documentation of a comprehensive family cancer history typically includes the following:
- A three-generation pedigree consisting of a minimum of first- and second-degree relatives on both the maternal and paternal sides of the family. Information on multiple generations helps to demonstrate inheritance patterns. Hereditary cancer can be inherited from either the maternal or the paternal side of the family, even in sex-limited (e.g., prostate cancer) and sex-influenced (e.g., breast cancer) phenotypes.
- Race, ancestry, and ethnicity of all grandparents. This may influence decisions about genetic testing because specific pathogenic variants in some genes are known to occur with increased frequency in some populations (founder effect).
- Information about seemingly unrelated conditions, such as birth defects, atypical skin bumps, or other nonmalignant conditions of children and adults that may aid in the diagnosis of a cancer susceptibility syndrome.
- Notation of adoption, nonpaternity (the biologic father should be included in the pedigree), consanguinity, and use of assisted reproductive technology (e.g., donor egg or sperm).
- Distinguish gender from sex in the pedigree when they are discordant.
A three-generation family history includes the following:
- First-degree relatives (e.g., children, brothers and sisters, and parents).
- Second-degree relatives (e.g., grandparents, aunts and uncles, nieces and nephews, grandchildren, and half-siblings).
- Third-degree relatives (e.g., first cousins, great aunts, and great uncles).
- Additional distant relatives are included if information is available, especially when there are known cancer histories among them.
For any relative with cancer, collect the following information:
- Primary site of each cancer. Obtaining medical documentation of key cancers (e.g., pathology reports, clinical documents, and death certificates) is especially relevant to risk assessment and/or management recommendations.
- Age at diagnosis for each primary cancer.
- Where the relative was diagnosed and/or treated.
- History of surgery or treatments that may have reduced the risk of cancer. For example, bilateral salpingo-oophorectomy in a premenopausal woman significantly reduces the risk of ovarian and breast cancers. This may mask underlying hereditary predisposition to these cancers.
- Current age (if living).
- Age at death and cause of death (if deceased).
- Carcinogenic exposures (e.g., alcohol and tobacco use, sun exposure, radiation exposure, asbestos exposure) or other known cancer site-specific risk factors.
- Prior germline genetic testing results.
- Other significant health problems.
- For more information, see the Accuracy of the family history section.
For relatives not affected with cancer, collect the following information:
- Current age or age at death.
- Cause of death (if deceased).
- History of any surgeries or treatments that may have reduced the risk of cancer.
- Cancer screening practices.
- Any nonmalignant features associated with the syndrome in question.
- Carcinogenic exposures (e.g., alcohol and tobacco use, sun exposure, radiation exposure, asbestos exposure) or other known cancer site-specific risk factors.
- Prior germline genetic testing results.
- Prior tumor testing results (including genomic profiling).
- Other significant health problems.
Accuracy of the family history
The accuracy of the family history has a direct bearing on determining the differential diagnoses, selecting appropriate testing, interpreting results of the genetic tests, refining individual cancer risk estimates, and outlining screening and risk reduction recommendations. People often have incomplete or inaccurate information about the cancer history in their families. In a nationally representative sample of 3,504 adults from the United States, only 31% of participants stated that they knew the cancers in their relatives very well. Accuracy of family history reporting varied by cancer site and a relative's degree of relatedness to the patient. Reporting of cancer family histories was the most accurate for breast cancer cases and less accurate for gynecologic and colon cancer cases.
Self-reported family histories may contain errors, and in rare instances, they can be fictitious. Obtaining a pathology report is the most reliable way to document a cancer's histology. Verification of cancers in a family history can also be achieved via other medical records, tumor registries, and/or death certificates. Patient education has been shown to improve the completeness of family history collection and may lead to more-accurate risk stratification, referrals for genetic counseling, and changes to management recommendations. Confirming the primary site of cancers in the family that will affect the calculation of hereditary predisposition probabilities and/or estimation of empiric cancer risks may be important, especially if decisions about treatments such as risk-reducing surgery will be based on this family history.
Determining Cancer Risk
Analysis of the family history
Because a family history of cancer is one of the important predictors of cancer risk, analysis of the pedigree constitutes an important aspect of risk assessment. This analysis might be thought of as a series of the following questions:
- What evidence suggests that a cancer susceptibility syndrome is present in this family?
- If a syndrome is suspected, what are the differential diagnoses?
- What factors can make the family history difficult to interpret?
- What is the most likely mode of inheritance for a hereditary cancer syndrome?
- What is a family member's risk of developing cancer if an inherited susceptibility exists?
- If a recognizable hereditary cancer syndrome is not found, what is an individual's cancer risk based on other epidemiological risk factors?
- What evidence suggests that a cancer susceptibility syndrome is present in
this family?
Hereditary cancer syndromes are found by analyzing both pedigrees and physical findings. The index of suspicion is raised by the following:
- Multiple cancers in close relatives, particularly in multiple generations.
- Early age of cancer onset (younger than age 40 to 50 y for adult-onset cancers).
- Multiple primary cancers in a single individual.
- Bilateral cancers.
- Recognition of the known association between etiologically related cancers in the family (e.g., breast and ovarian cancers; colon and endometrial cancers).
- Presence of congenital anomalies or precursor lesions that are known to be associated with increased cancer risk (e.g., presence of atypical nevi and risk of malignant melanoma).
- Recognizable mendelian inheritance pattern.
- Specific tumor types or pathologies associated with germline pathogenic variants in cancer susceptibility genes, regardless of family history (e.g., ovarian cancer, medullary thyroid cancer, triple-negative breast cancer, sex cord tumors in ovarian cancer). For more information on these tumor types, see Genetics of Breast and Gynecologic Cancers and Genetics of Endocrine and Neuroendocrine Neoplasias.
- Abnormal results from colon or endometrial tumor testing with microsatellite instability or immunohistochemistry, suggestive of Lynch syndrome. For more information, see the Genetics of Lynch syndrome section in Genetics of Colorectal Cancer.
- Somatic mutations identified from tumor genomic profiling that may be present in the germline.
Clinical characteristics associated with different cancer genetic syndromes are summarized in the following comprehensive set of personal and family history criteria published by the American College of Medical Genetics and Genomics (ACMG) and the National Society of Genetic Counselors (NSGC). These practice guidelines address tumor types, other potential features, and related criteria that would prompt a genetics referral. ACMG and NSGC state that the guidelines are intended to maximize appropriate referral of at-risk individuals for cancer genetics consultations, but they are not meant to provide genetic testing or treatment recommendations. However, these organizations also note the importance of consulting other guidelines that are more frequently updated, like the National Comprehensive Cancer Network.
- If a syndrome is suspected, what are the differential diagnoses?
The most common indications for genetic counseling/testing are suspected hereditary breast cancer or hereditary colon cancer syndromes.
For hereditary breast cancer, genetic counseling and testing criteria are broad. Multigene panel testing has revealed that pathogenic variants in several other high- and moderate-penetrance genes other than BRCA1 and BRCA2 contribute to this phenotype, such as PALB2, CHEK2, and ATM.
Differential diagnoses for hereditary colon cancer syndromes are based on several factors, including the following: number of colorectal polyps, type of colorectal polyps, histopathology of gastrointestinal malignancies, and histopathology of other malignancies. However, in the absence of polyposis and rare pathologies, Lynch syndrome is often on the differential diagnosis list. Furthermore, Lynch syndrome may be on the differential diagnosis list when there are cases of breast and/or ovarian cancer in the family that are not consistent with hereditary breast and ovarian cancer. For more information, see the Lynch syndrome section in Genetics of Colorectal Cancer.
Diagnostic and testing criteria exist for several rare syndromes such as Li-Fraumeni syndrome, Cowden syndrome, multiple endocrine neoplasias, and familial adenomatous polyposis. In some cases, pathognomonic features are indicators for rare cancer syndromes.
Based on these considerations, genetic testing options include targeted testing for pathogenic variants in one or several genes or testing with a large gene panel.
- What factors can make the family history difficult to interpret?
Other factors may complicate recognition of basic inheritance patterns or represent different types of disease etiology.
Common examples of factors that complicate family history structure include the following:
- Small family size.
- Incomplete information due to a lack of family history knowledge (e.g., due to adoption or lack of information about cancers in relatives).
- Gender imbalance (e.g., few women in a family suspected of hereditary breast cancer).
- Deaths at particularly early ages.
- Removal of the at-risk organ, either for risk reduction or as a result of a medical condition (e.g., hysterectomy due to history of uterine fibroids or endometriosis may hamper the identification of Lynch syndrome).
- Misattributed parentage.
- Consanguinity.
Genetic factors that may affect family history interpretation include:
- Late or variable onset of disease.
- Nonpenetrance.
- Variable expression.
- Genetic heterogeneity.
- De novo pathogenic variant.
- Mosaicism (somatic or germline).
- What is the most likely mode of inheritance for a hereditary cancer syndrome?
The mode of inheritance refers to the way that genetic traits are transmitted in a family.
Most modes of inheritance are established when the patient is given a clinical diagnosis and when a compatible, but not necessarily conclusive, inheritance pattern is seen on a pedigree. Most recognized hereditary cancer syndromes are inherited in anautosomal dominant or an autosomal recessive fashion. Clues to recognizing these patterns within a pedigree are described below.
- Autosomal dominant inheritance refers to disorders that are expressed in heterozygotes (i.e., the affected person has one copy of a pathogenic variant and one
normal copy of the gene). All of the major hereditary breast/gynecological and colorectal cancer syndromes are inherited in an autosomal dominant fashion; these syndromes include the following: BRCA1- and BRCA2-associated hereditary breast and gynecologic cancers, Li-Fraumeni syndrome, Cowden syndrome, Lynch syndrome. Autosomal dominant inheritance is
characterized by the following factors:
- Vertical occurrence (i.e., syndrome-associated cancers are seen in successive generations).
- Syndrome-associated cancers are seen only on one side of the family (i.e., unipaternal or unimaternal).
- Males and females may inherit and transmit the disorder to offspring.
- Male-to-male transmission may be seen.
- Offspring have a 50% chance of inheriting a pathogenic variant and a 50% chance of inheriting a normal copy of the gene.
- The condition may appear to skip a generation due to several factors: incomplete penetrance, early death, delayed age of disease onset, or a small number of affected males/females when the at-risk organ is gender-specific (e.g., prostate and ovary).
- It is possible for an individual to have a de novo (new) pathogenic variant. This person would be the first affected member of his or her family, but he/she could transmit this pathogenic variant to his/her offspring in an autosomal dominant fashion.
- It is possible for an individual to have pathogenic variants in more than one gene associated with known autosomal dominant inherited cancer predisposition syndromes. In families with phenotypes suggestive of more than one type of hereditary cancer syndrome, identifying multiple pathogenic variants helps better explain complex personal and/or family histories of cancer. This can help determine the appropriate testing strategy for family members.
- In autosomal recessive inheritance, an
affected person must be homozygous (i.e., an individual has two pathogenic variants and inherited one
pathogenic variant from each parent). Well-defined cancer susceptibility syndromes with autosomal recessive inheritance patterns include the following: Bloom syndrome, ataxia telangiectasia, MUTYH-associated polyposis, and Fanconi anemia. Autosomal recessive inheritance is characterized by the following:
- Horizontal occurrence (i.e., syndrome-associated cancers are only seen in one generation; affected siblings are seen in the absence of affected parents). This is generally not seen in successive generations.
- Pathogenic variants are inherited from both sides of the family (i.e., biparental inheritance).
- Parents are heterozygouscarriers; each parent carries one pathogenic variant and one functional copy of the gene.
- Parents usually do not express the features associated with the pathogenic variant that they carry; in some cases, parents may show a mild version of some features.
- When two parents are heterozygous, there is a 25% risk for future offspring to be affected (homozygous).
- Most cancers, and most familial cancers, appear to have a complex etiology. Within clinical settings, negative or uninformative genetic testing results are common. Multiple factors may contribute to the development of the observed cancer(s); these factors may be difficult to pinpoint.
- Complex or multifactorial disease inheritance is used to describe conditions caused by genetic and environmental factors. In contrast to mendelian diseases, where carrying one pathogenic variant is associated with a high likelihood for developing the disease, complex/multifactorial diseases are caused by the interaction of genes and environmental factors. Therefore, a single genetic locus is not responsible for the condition. In most cases, the combined effects of genetic, lifestyle, and environmental factors determine a person’s likelihood of being affected with a condition, such as cancer.
Clustering of cancer among relatives is common. However, determining the underlying causes of this is difficult when there is not a clear pattern of cancer in the family. Individuals with common malignancies, like lung cancer, can have many relatives with cancer. These familial aggregations are thought to be caused by a combination of the following: exposures to known carcinogens (such as tobacco smoke), pathogenic variants in high-penetrance cancer risk genes, and alterations in low-penetrance genes that affect the metabolism of carcinogens.
The general practitioner is likely to encounter some families with strong genetic predispositions to cancer. Recognizing an individual's cancer susceptibility may have dramatic consequences for his/her health and management. Although some high-risk pathogenic variants in major cancer susceptibility genes are consistent with recognizable mendelian inheritance patterns, these syndromes are rare.
- Autosomal dominant inheritance refers to disorders that are expressed in heterozygotes (i.e., the affected person has one copy of a pathogenic variant and one
normal copy of the gene). All of the major hereditary breast/gynecological and colorectal cancer syndromes are inherited in an autosomal dominant fashion; these syndromes include the following: BRCA1- and BRCA2-associated hereditary breast and gynecologic cancers, Li-Fraumeni syndrome, Cowden syndrome, Lynch syndrome. Autosomal dominant inheritance is
characterized by the following factors:
- What is a family member's risk of developing cancer if an inherited susceptibility
exists?
These probabilities vary by syndrome, family, gene, and pathogenic variant. Different pathogenic variants in the same gene can confer varying cancer risks, or the same pathogenic variant can be associated with different clinical manifestations in separate families. Many of these scenarios can be attributed to penetrance and expressivity. For more information, see the Penetrance of Inherited Susceptibility to Hereditary Breast and/or Gynecologic Cancers section in Genetics of Breast and Gynecologic Cancers.
- If a recognizable hereditary cancer syndrome is not found, what is an individual's cancer risk based on other
epidemiological risk factors?
A positive family history may provide information about cancer risk, even when a hereditary cancer syndrome is not found. For example, the risk of having a single relative affected with breast or colorectal cancer can be estimated from epidemiologic data and family studies. For more information, see Genetics of Breast and Gynecologic Cancers and Genetics of Colorectal Cancer.
Methods of quantifying cancer risk
The overarching goal of cancer risk assessment is to individualize cancer risk management recommendations based on personalized risk. Methods to calculate risk utilize health history information and risk factor and family history data often in combination with emerging biologic and genetic/genomic evidence to establish predictions. Multiple methodologies are used to calculate risk, including statistical models, prevalence data from specific populations, penetrance data when a documented pathogenic variant has been identified in a family, mendelian inheritance, and Bayesian analysis. All models have distinct capabilities, weaknesses, and limitations based on the methodology, sample size, and/or population used to create the model. Methods to individually quantify risk encompass two primary areas: the probability of harboring a pathogenic variant in a cancer susceptibility gene and the risk of developing a specific form of cancer.
Risk of harboring a pathogenic variant in a cancer susceptibility gene
The decision to offer genetic testing for cancer susceptibility is complex and can be aided in part by objectively assessing an individual's and/or family's probability of harboring a pathogenic variant. Predicting the probability of harboring a pathogenic variant in a cancer susceptibility gene can be done using several strategies, including empiric data, statistical models, population prevalence data, Mendel’s laws, Bayesian analysis, and specific health information, such as tumor-specific features. All of these methods are gene specific or cancer-syndrome specific and are employed only after a thorough assessment has been completed and genetic differential diagnoses have been established.
If a gene or hereditary cancer syndrome is suspected, syndrome-specific models can be used to determine if genetic testing is warranted. Models and prevalence data are most effective when they are applied to the populations that are best suited for their use. For instance, a model or prevalence data derived from a population study of individuals older than 35 years may not accurately be applied in a population aged 35 years and younger. Care must be taken when interpreting the data obtained from various risk models because they differ with regard to what is actually being estimated. Some models estimate the risk of a pathogenic variant being present in the family; others estimate the risk of a pathogenic variant being present in the individual being counseled. Some models estimate the risk of specific cancers developing in an individual, while others estimate more than one of the data above. Other important considerations include critical family constructs, which can significantly impact model reliability, such as small family size or male-dominated families when the cancer risks are predominantly female in origin, adoption, and early deaths from other causes. In addition, most models provide gene and/or syndrome-specific probabilities but do not account for the possibility that the personal and/or family history of cancer may be conferred by an as-yet-unidentified cancer susceptibility gene. In the absence of a documented pathogenic variant in the family, critical assessment of the personal and family history is essential in determining the usefulness and limitations of probability estimates used to aid in the decisions regarding indications for genetic testing.
For more information on syndrome-specific models, see the following PDQ summaries:
- Genetics of Breast and Gynecologic Cancers
- Genetics of Colorectal Cancer
- Genetics of Skin Cancer
When a pathogenic variant has been identified in a family and a test report documents that finding, prior probabilities can be ascertained with a greater degree of reliability. In this setting, probabilities can be calculated based on the pattern of inheritance associated with the gene in which the pathogenic variant has been identified. In addition, critical to the application of mendelian inheritance is the consideration of integrating Bayes Theorem, which incorporates other variables, such as current age, into the calculation for a more accurate posterior probability. This is especially useful in individuals who have lived to be older than the age at which cancer is likely to develop based on the pathogenic variant identified in their family and therefore have a lower likelihood of harboring the family pathogenic variant when compared with the probability based on their relationship to the carrier in the family.
Even in the case of a documented pathogenic variant on one side of the family, careful assessment and evaluation of the individual’s personal and family history of cancer is essential to rule out cancer risk or suspicion of a cancer susceptibility gene pathogenic variant on the other side of the family (maternal or paternal, as applicable). Segregation of more than one pathogenic variant in a family is possible (e.g., in circumstances in which a cancer syndrome has founder pathogenic variants associated with families of particular ancestral origin).
Risk of developing cancer
Unlike pathogenic variant probability models that predict the likelihood that a given personal and/or family history of cancer could be associated with a pathogenic variant in a specific gene(s), other methods and models can be used to estimate the risk of developing cancer over time. Similar to pathogenic variant probability assessments, cancer risk calculations are also complex and necessitate a detailed health history and family history. In the presence of a documented pathogenic variant, cancer risk estimates can be derived from peer-reviewed penetrance data. Penetrance data are constantly being refined and many genetic variants have variable penetrance because other variables may impact the absolute risk of cancer in any given patient. Modifiers of cancer risk in carriers of pathogenic variants include the variant's effect on the function of the gene/protein (e.g., variant type and position), the contributions of modifier genes, and personal and environmental factors (e.g., the impact of bilateral salpingo-oophorectomy performed for other indications in a woman who harbors a BRCA pathogenic variant). When there is evidence of an inherited susceptibility to cancer but genetic testing has not been performed, analysis of the pedigree can be used to estimate cancer risk. This type of calculation uses the probability the individual harbors a genetic variant and variant-specific penetrance data to calculate cancer risk.
In the absence of evidence of a hereditary cancer syndrome, several methods can be utilized to estimate cancer risk. Relative risk data from studies of specific risk factors provide ratios of observed versus expected cancers associated with a given risk factor. However, utilizing relative risk data for individualized risk assessment can have significant limitations: relative risk calculations will differ based on the type of control group and other study-associated biases, and comparability across studies can vary widely. In addition, relative risks are lifetime ratios and do not provide age-specific calculations, nor can the relative risk be multiplied by population risk to provide an individual's risk estimate.
In spite of these limitations, disease-specific cumulative risk estimates are most often employed in clinical settings. These estimates usually provide risk for a given time interval and can be anchored to cumulative risks of other health conditions in a given population (e.g., the 5-year risk by the Gail model). Cumulative risk models have limitations that may underestimate or overestimate risk. For example, the Gail model excludes paternal family histories of breast cancer. Furthermore, many of these models were constructed from data derived from predominantly white populations and may have limited validity when used to estimate risk in other ethnicities.
Cumulative risk estimates are best used when evidence of other underlying significant risk factors have been ruled out. Careful evaluation of an individual's personal health and family history can identify other confounding risk factors that may outweigh a risk estimate derived from a cumulative risk model. For example, a woman with a prior biopsy showing lobular carcinoma in situ (LCIS) whose mother was diagnosed with breast cancer at age 65 years has a greater lifetime risk from her history of LCIS than her cumulative lifetime risk of breast cancer based on one first-degree relative. In this circumstance, recommendations for cancer risk management would be based on the risk associated with her LCIS. Unfortunately, there is no reliable method for combining all of an individual's relevant risk factors for an accurate absolute cancer risk estimate, nor are individual risk factors additive.
In summary, careful ascertainment and review of personal health and cancer family history are essential adjuncts to the use of prior probability models and cancer risk assessment models to assure that critical elements influencing risk calculations are considered. Influencing factors include the following:
- Differential diagnosis that is consistent with the personal and cancer family history.
- Consideration of factors that influence how informative the family history may be.
- Population that is best suited for the use of the model.
- Tumor-specific features that may be suspicious for an inherited predisposition or modify individual cancer risk predictions.
- Model-specific limitations that can overestimate or underestimate calculations.
A number of investigators are developing health care provider decision support tools such as the Genetic Risk Assessment on the Internet with Decision Support (GRAIDS), but at this time, clinical judgment remains a key component of any prior probability or absolute cancer risk estimation.
References
- Robson ME, Bradbury AR, Arun B, et al.: American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. J Clin Oncol 33 (31): 3660-7, 2015.
- Berliner JL, Cummings SA, Boldt Burnett B, et al.: Risk assessment and genetic counseling for hereditary breast and ovarian cancer syndromes-Practice resource of the National Society of Genetic Counselors. J Genet Couns 30 (2): 342-360, 2021.
- Holter S, Hall MJ, Hampel H, et al.: Risk assessment and genetic counseling for Lynch syndrome - Practice resource of the National Society of Genetic Counselors and the Collaborative Group of the Americas on Inherited Gastrointestinal Cancer. J Genet Couns 31 (3): 568-583, 2022.
- Lancaster JM, Powell CB, Chen LM, et al.: Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol 136 (1): 3-7, 2015.
- Committee on Practice Bulletins–Gynecology, Committee on Genetics, Society of Gynecologic Oncology: Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol 130 (3): e110-e126, 2017.
- Rimer BK, Schildkraut JM, Lerman C, et al.: Participation in a women's breast cancer risk counseling trial. Who participates? Who declines? High Risk Breast Cancer Consortium. Cancer 77 (11): 2348-55, 1996.
- Evans DG, Burnell LD, Hopwood P, et al.: Perception of risk in women with a family history of breast cancer. Br J Cancer 67 (3): 612-4, 1993.
- Kash KM, Holland JC, Halper MS, et al.: Psychological distress and surveillance behaviors of women with a family history of breast cancer. J Natl Cancer Inst 84 (1): 24-30, 1992.
- Davis S, Stewart S, Bloom J: Increasing the accuracy of perceived breast cancer risk: results from a randomized trial with Cancer Information Service callers. Prev Med 39 (1): 64-73, 2004.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 2.2024. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023. Available online with free registration. Last accessed October 31, 2023.
- Kuzbari Z, Bandlamudi C, Loveday C, et al.: Germline-focused analysis of tumour-detected variants in 49,264 cancer patients: ESMO Precision Medicine Working Group recommendations. Ann Oncol 34 (3): 215-227, 2023.
- Li X, Kahn RM, Wing N, et al.: Leveraging Health Information Technology to Collect Family Cancer History: A Systematic Review and Meta-Analysis. JCO Clin Cancer Inform 5: 775-788, 2021.
- Tehranifar P, Wu HC, Shriver T, et al.: Validation of family cancer history data in high-risk families: the influence of cancer site, ethnicity, kinship degree, and multiple family reporters. Am J Epidemiol 181 (3): 204-12, 2015.
- Bennett RL, Steinhaus KA, Uhrich SB, et al.: Recommendations for standardized human pedigree nomenclature. Pedigree Standardization Task Force of the National Society of Genetic Counselors. Am J Hum Genet 56 (3): 745-52, 1995.
- Bennett RL, French KS, Resta RG, et al.: Standardized human pedigree nomenclature: update and assessment of the recommendations of the National Society of Genetic Counselors. J Genet Couns 17 (5): 424-33, 2008.
- Bennett RL, French KS, Resta RG, et al.: Practice resource-focused revision: Standardized pedigree nomenclature update centered on sex and gender inclusivity: A practice resource of the National Society of Genetic Counselors. J Genet Couns 31 (6): 1238-1248, 2022.
- Lu KH, Wood ME, Daniels M, et al.: American Society of Clinical Oncology Expert Statement: collection and use of a cancer family history for oncology providers. J Clin Oncol 32 (8): 833-40, 2014.
- Schneider K: Collection and interpretation of cancer histories. In: Schneider KA: Counseling About Cancer: Strategies for Genetic Counseling. 2nd ed. Wiley-Liss, 2002, pp 129-166.
- Mitchell RJ, Brewster D, Campbell H, et al.: Accuracy of reporting of family history of colorectal cancer. Gut 53 (2): 291-5, 2004.
- Schneider KA, DiGianni LM, Patenaude AF, et al.: Accuracy of cancer family histories: comparison of two breast cancer syndromes. Genet Test 8 (3): 222-8, 2004.
- Douglas FS, O'Dair LC, Robinson M, et al.: The accuracy of diagnoses as reported in families with cancer: a retrospective study. J Med Genet 36 (4): 309-12, 1999.
- Sijmons RH, Boonstra AE, Reefhuis J, et al.: Accuracy of family history of cancer: clinical genetic implications. Eur J Hum Genet 8 (3): 181-6, 2000.
- Mai PL, Garceau AO, Graubard BI, et al.: Confirmation of family cancer history reported in a population-based survey. J Natl Cancer Inst 103 (10): 788-97, 2011.
- Ozanne EM, O'Connell A, Bouzan C, et al.: Bias in the reporting of family history: implications for clinical care. J Genet Couns 21 (4): 547-56, 2012.
- Brennan P, Claber O, Brennan T: Cancer family history triage: a key step in the decision to offer screening and genetic testing. Fam Cancer 12 (3): 497-502, 2013.
- Krakow M, Rising CJ, Trivedi N, et al.: Prevalence and Correlates of Family Cancer History Knowledge and Communication Among US Adults. Prev Chronic Dis 17: E146, 2020.
- Qureshi N, Wilson B, Santaguida P, et al.: Collection and Use of Cancer Family History in Primary Care. Evidence Report/Technology Assessment No. 159. Agency for Healthcare Research and Quality, 2007. AHRQ Pub No. 08-E001.
- Murff HJ, Spigel DR, Syngal S: Does this patient have a family history of cancer? An evidence-based analysis of the accuracy of family cancer history. JAMA 292 (12): 1480-9, 2004.
- John EM, Canchola AJ, Sangaramoorthy M, et al.: Race/Ethnicity and Accuracy of Self-Reported Female First-Degree Family History of Breast and Other Cancers in the Northern California Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev 28 (11): 1792-1801, 2019.
- Evans DG, Kerr B, Cade D, et al.: Fictitious breast cancer family history. Lancet 348 (9033): 1034, 1996.
- Beadles CA, Ryanne Wu R, Himmel T, et al.: Providing patient education: impact on quantity and quality of family health history collection. Fam Cancer 13 (2): 325-32, 2014.
- Hampel H, Bennett RL, Buchanan A, et al.: A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 17 (1): 70-87, 2015.
- Bashford MT, Kohlman W, Everett J, et al.: Addendum: A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 21 (12): 2844, 2019.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Colorectal. Version 1.2023. Plymouth Meeting, PA: National Comprehensive Cancer Network, 2023. Available with free registration. Last accessed June 28, 2023.
- Spoto CPE, Gullo I, Carneiro F, et al.: Hereditary gastrointestinal carcinomas and their precursors: An algorithm for genetic testing. Semin Diagn Pathol 35 (3): 170-183, 2018.
- Roberts ME, Jackson SA, Susswein LR, et al.: MSH6 and PMS2 germ-line pathogenic variants implicated in Lynch syndrome are associated with breast cancer. Genet Med 20 (10): 1167-1174, 2018.
- Espenschied CR, LaDuca H, Li S, et al.: Multigene Panel Testing Provides a New Perspective on Lynch Syndrome. J Clin Oncol 35 (22): 2568-2575, 2017.
- Bougeard G, Renaux-Petel M, Flaman JM, et al.: Revisiting Li-Fraumeni Syndrome From TP53 Mutation Carriers. J Clin Oncol 33 (21): 2345-52, 2015.
- Pilarski R, Eng C: Will the real Cowden syndrome please stand up (again)? Expanding mutational and clinical spectra of the PTEN hamartoma tumour syndrome. J Med Genet 41 (5): 323-6, 2004.
- Eng C: PTEN Hamartoma Tumor Syndrome (PHTS). In: Adam MP, Feldman J, Mirzaa GM, et al., eds.: GeneReviews. University of Washington, Seattle, 1993-2024, pp. Available online. Last accessed March 6, 2024.
- Brandi ML, Gagel RF, Angeli A, et al.: Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86 (12): 5658-71, 2001.
- Weitzel JN, Lagos VI, Cullinane CA, et al.: Limited family structure and BRCA gene mutation status in single cases of breast cancer. JAMA 297 (23): 2587-95, 2007.
- Kauff ND, Offit K: Modeling genetic risk of breast cancer. JAMA 297 (23): 2637-9, 2007.
- Kramer JL, Velazquez IA, Chen BE, et al.: Prophylactic oophorectomy reduces breast cancer penetrance during prospective, long-term follow-up of BRCA1 mutation carriers. J Clin Oncol 23 (34): 8629-35, 2005.
- Harper PS: Practical Genetic Counselling. 3rd ed. Wright, 1988.
- Whitworth J, Skytte AB, Sunde L, et al.: Multilocus Inherited Neoplasia Alleles Syndrome: A Case Series and Review. JAMA Oncol 2 (3): 373-9, 2016.
- Stratton MR: Exploring the genomes of cancer cells: progress and promise. Science 331 (6024): 1553-8, 2011.
- Freedman AN, Seminara D, Gail MH, et al.: Cancer risk prediction models: a workshop on development, evaluation, and application. J Natl Cancer Inst 97 (10): 715-23, 2005.
- Lindor NM, Lindor RA, Apicella C, et al.: Predicting BRCA1 and BRCA2 gene mutation carriers: comparison of LAMBDA, BRCAPRO, Myriad II, and modified Couch models. Fam Cancer 6 (4): 473-82, 2007.
- Domchek SM, Eisen A, Calzone K, et al.: Application of breast cancer risk prediction models in clinical practice. J Clin Oncol 21 (4): 593-601, 2003.
- Riley BD, Culver JO, Skrzynia C, et al.: Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J Genet Couns 21 (2): 151-61, 2012.
- Offit K, Brown K: Quantitating familial cancer risk: a resource for clinical oncologists. J Clin Oncol 12 (8): 1724-36, 1994.
- Apicella C, Andrews L, Hodgson SV, et al.: Log odds of carrying an Ancestral Mutation in BRCA1 or BRCA2 for a Defined personal and family history in an Ashkenazi Jewish woman (LAMBDA). Breast Cancer Res 5 (6): R206-16, 2003.
- Chenevix-Trench G, Milne RL, Antoniou AC, et al.: An international initiative to identify genetic modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: the Consortium of Investigators of Modifiers of BRCA1 and BRCA2 (CIMBA). Breast Cancer Res 9 (2): 104, 2007.
- Hoskins KF, Stopfer JE, Calzone KA, et al.: Assessment and counseling for women with a family history of breast cancer. A guide for clinicians. JAMA 273 (7): 577-85, 1995.
- Adams-Campbell LL, Makambi KH, Palmer JR, et al.: Diagnostic accuracy of the Gail model in the Black Women's Health Study. Breast J 13 (4): 332-6, 2007 Jul-Aug.
- Fisher ER, Land SR, Fisher B, et al.: Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: twelve-year observations concerning lobular carcinoma in situ. Cancer 100 (2): 238-44, 2004.
- Chuba PJ, Hamre MR, Yap J, et al.: Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: analysis of surveillance, epidemiology, and end results data. J Clin Oncol 23 (24): 5534-41, 2005.
- Emery J, Morris H, Goodchild R, et al.: The GRAIDS Trial: a cluster randomised controlled trial of computer decision support for the management of familial cancer risk in primary care. Br J Cancer 97 (4): 486-93, 2007.
The Option of Genetic Testing
Factors to Consider When Offering Testing
Indications for testing
Experts recommend offering genetic testing when a risk assessment suggests the presence of an inherited cancer syndrome for which specific genes have been identified. The American Society of Clinical Oncology (ASCO) Policy Statement on Genetic Testing for Cancer Susceptibility proposes that genetic testing be offered when the following conditions apply:
- An individual has a personal or family history suggestive of a genetic cancer susceptibility syndrome.
- The results of the test can be interpreted.
- Testing will influence medical management.
Characteristics used in making this determination are discussed in the PDQ summaries on the genetics of specific cancers. Even when individual and family history characteristics indicate a possible inherited cancer syndrome, individuals may elect not to proceed with testing after discussion of potential risks, benefits, and limitations, as discussed below. Conversely, individuals whose pedigrees are incomplete or uninformative due to very small family size, early deaths, or incomplete data on key family members may elect to pursue genetic testing in an attempt to better define their risk status. In these situations, it is particularly important that the pretest counseling fully explore the limitations of the testing process.
ASCO's 2010 and 2015 policy statements addressed testing for low- to moderate-penetrance genes and direct-to-consumer testing.
ASCO’s position is that when a test, regardless of clinical utility, is ordered by a health care professional, the provider is responsible for organizing follow-up care based on the findings. For tests that are ordered by the consumer without health care professional involvement, management decisions are based on the evidence for clinical utility. For tests with accepted clinical utility, follow-up care can be guided by the evidence for cancer risk associated with the genetic test finding. However, in tests ordered by the consumer that have uncertain clinical utility, ASCO recommends that follow-up care consist of education regarding the lack of evidence regarding the test's clinical utility and that cancer risk management decisions be guided by established cancer risk factors.
In 2015, ASCO updated its policy to address the challenges of new technologies in cancer genetics, including multigene (panel) testing for cancer genetic susceptibility, as well as incidental germline findings from somatic mutation profiling. ASCO's statement supports informing patients about medically relevant germline findings discovered during somatic mutation profiling.
Genetic education and counseling approaches (including the interpretation of genetic test results) will vary if genetic testing has already been attempted (for more information, see Figure 1). In general, there are two primary circumstances in which genetic testing is performed:
- Families with evidence of an inherited susceptibility that have not had any genetic testing or in which genetic testing has not identified a pathogenic variant.
- Families with a documented pathogenic variant.
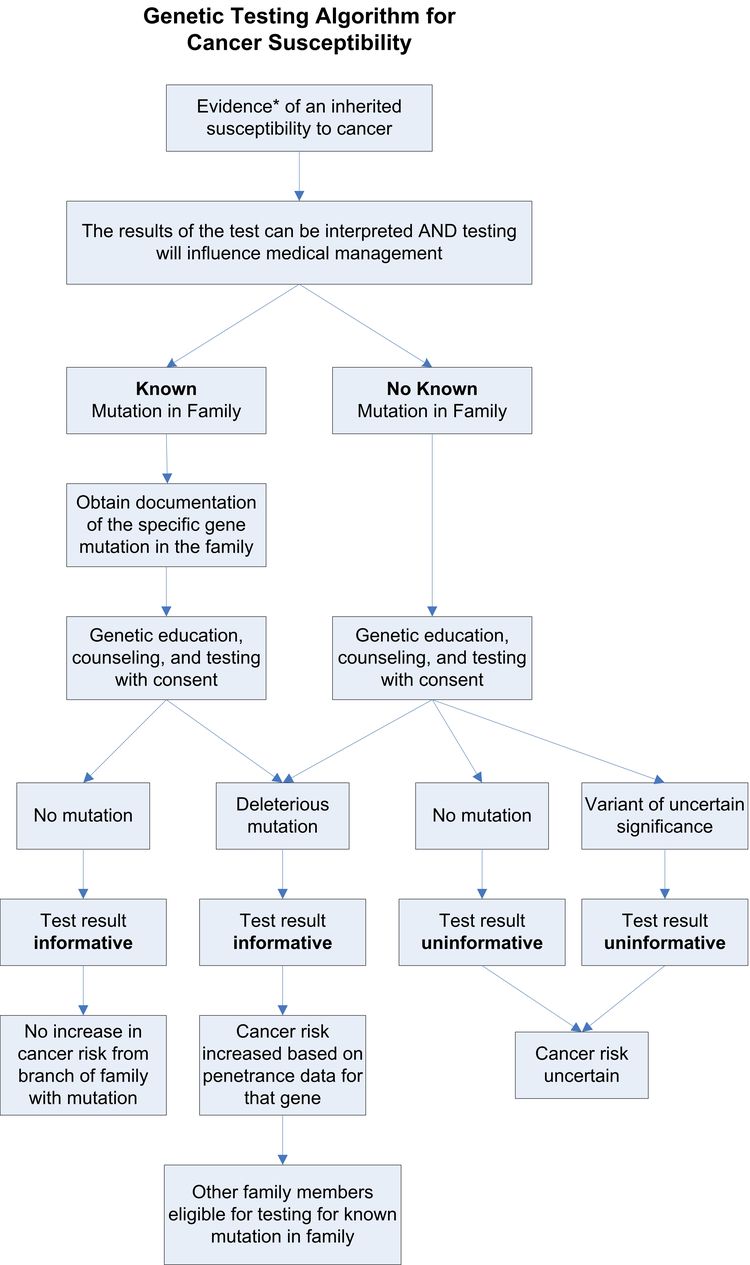 Figure 1. This genetic testing algorithm depicts the multistep process of testing for cancer susceptibility.
Figure 1. This genetic testing algorithm depicts the multistep process of testing for cancer susceptibility.
Value of testing an affected family member first
Genetic susceptibility testing generally yields the most useful information when a living family member with the cancer of concern is tested before other family members; this can help determine if his/her cancer has a genetic origin. If testing is deferred while follow-up with an affected relative is pending, consider providing interim cancer risk management guidelines to the unaffected proband. Possible genetic testing outcomes include the following (for more information, see Figure 1):
- Pathogenic variant detected.
- No variant detected.
- Variant of uncertain significance (VUS) detected.
If a documented pathogenic variant (associated with cancer risk) is identified, risks are based on penetrance data for pathogenic variants of that specific gene. In addition, other family members may be tested for the presence or absence of this specific pathogenic variant. If no variant is found in an affected family member, testing is considered uninformative and thus there is no basis for testing unaffected relatives. Failure of the laboratory to detect a pathogenic variant in an affected family member does not rule out an inherited basis for the cancer in that family. Reasons why testing could be uninformative include the following:
- The cancer in the family may be associated with a cancer susceptibility gene other than the gene that was tested.
- The cancer in the family may be associated with a pathogenic variant, but the cancer in the specific family member who underwent testing is not associated with that variant. This can occur especially with cancers that are common in the general population, such as breast cancer or prostate cancer. The family member who is affected with the disease but is not a carrier of the pathogenic variant associated with the inherited predisposition to cancer in the family is considered a phenocopy.
- Identifying a genetic variant may not be possible given the limited sensitivity of the laboratory techniques used to detect genetic variants. There may be additional testing available to detect certain types of variants that would have been missed by the initial genetic test.
- The function of the gene could be altered by a pathogenic variant in a different gene.
Lastly, testing may reveal a VUS. This result means that a genetic variant has been found; however, the extent that this variant increases cancer risk, or whether it is associated with the history of cancer in the family, is uncertain. In this circumstance, some clues as to the significance of the variant can be derived from the following:
- The location of the variant in relation to regions and function of a gene.
- The specific change; since many variants are missense variants, not all amino acid substitutions are as significant.
- Whether the variant has been documented in the presence of a documented pathogenic variant.
- Whether the variant is associated with the branch in the family with the cancer and/or whether the variant tracks with the cancers in the family.
Unfortunately, even with this information, there is often insufficient evidence to document the significance of a specific variant, and further clarifying research is required.
If there is no close, living, affected relative to undergo testing, or the living affected relative declines testing, other options may be discussed with the patient and the testing laboratory. In rare instances, if proper authorization is secured from the family, testing the stored tissue of a deceased relative may be considered. However, genetic tests done on stored tissue are technically difficult and may not yield a definitive result. Therefore, testing an unaffected person without prior testing of an affected family member may be performed. In these instances, counseling includes discussing that a negative test result does not rule out the presence of a cancer susceptibility gene in the family or in the patient and may be uninformative.
Testing in families with a documented pathogenic variant
Genetic testing for a documented familial pathogenic variant can be informative and will yield one of the following results (for more information, see Figure 1):
- Positive for the familial pathogenic variant.
- Negative for the familial pathogenic variant.
If the familial pathogenic variant is detected in a family member, their cancer risks are based on penetrance data for pathogenic variants in that specific gene. If the documented pathogenic variant is not found in a family member, the risk of cancer in that individual is equivalent to cancer risk in the general population. However, other risk factors and family history from the side of the family not associated with the documented pathogenic variant may increase the cancer risk above the general population levels.
In summary, genetic education and counseling includes identifying the most informative person in the family to test, which may be an affected family member rather than the individual seeking genetic services. In addition, counseling includes a discussion of the limitations of the test, all possible test outcomes, and the consequences of identifying a VUS.
Insurance coverage
Insurance coverage varies for cancer susceptibility testing, including multigene (panel) testing. In general, most individuals who meet specific criteria (e.g., National Comprehensive Cancer Network [NCCN] guidelines for BRCA1/BRCA2 or Lynch syndrome testing) are able to obtain insurance coverage for multigene testing. Of note, some insurance companies have contracts with specific laboratories through which testing must be ordered.
The Affordable Care Act (ACA) requires that private insurers cover—with no out-of-pocket costs to the insured—genetic counseling and BRCA1/BRCA2 testing for unaffected women meeting United States Preventive Services Task Force guidelines. Importantly, under ACA guidelines, women with a prior cancer diagnosis are not covered. The ACA does not stipulate that follow-up care based on genetic test results be covered (e.g., risk-reducing surgeries). However, some insurance companies require that pretest genetic counseling be performed by a credentialed genetics provider before testing is authorized. Before testing is ordered, it is important to verify costs and insurance coverage, including for Medicaid and Medicare patients. Medicare does not cover genetic testing if the patient has not had a cancer diagnosis associated with the pathogenic variants for which testing is ordered. In addition, unaffected individuals with Medicare are not covered for testing, even if they are tested for only a known familial pathogenic variant. Further, Medicare does not cover genetic counseling as a separately billable service. For individuals without insurance coverage and the underinsured, some laboratories offer low-cost options or have financial assistance programs.
Genetic testing and assisted reproductive technology
There is a risk of carriers passing on cancer-associated pathogenic variants to offspring. When an individual tests positive for one pathogenic variant in a cancer susceptibility gene, counseling about reproductive implications addresses not only the risks associated with autosomal dominant inheritance but also the potential risks of having a child with two pathogenic variants in the same gene (biallelic) that could result in a severe condition.
Assisted reproductive technology can be used for preimplantation genetic testing (PGT) and for prenatal cancer predisposition genetic testing using chorionic villus sampling and amniocentesis. For individuals with autosomal dominant cancer syndromes (e.g., those associated with APC, BRCA1/BRCA2, PTEN, or TP53 pathogenic variants), reproductive options exist for prenatal testing and PGT to detect offspring with one copy of the pathogenic variant (heterozygotes).
In some cases (e.g., carriers of germline pathogenic variants in ATM, BLM), assessing an individual’s partner's risk for carrying a pathogenic variant associated with a dominant or recessive syndrome (i.e., his or her personal and family history and ethnicity) is indicated. In the unlikely event that both parents are heterozygous for specific pathogenic variants, there is a 25% risk that a child will be homozygous and could have a severe phenotype. In light of this information, couples may consider PGT or prenatal testing.
A proposed analytic framework for counseling carriers about reproduction options includes consideration of the following issues:
- Does the cancer syndrome include childhood malignancies or significant morbidity or mortality at an early age?
- What is the penetrance associated with the genetic variant?
- How severe is the syndrome phenotype?
- Are there interventions available that decrease the pathogenic variant-associated cancer risk or are proven to detect cancer early when it is in a treatable form?
- Is there evidence of a different phenotype if an individual is a heterozygous or homozygous carrier?
In a study of 320 patients with different hereditary cancer syndromes, most were unaware of PGT; however, the majority expressed interest in learning more about the availability of PGT. Patients also preferred having a discussion about PGT with their genetic counselor or primary physician. Disease-specific factors (e.g., severity of the hereditary condition, quality of life, and medical interventions) and individual factors (e.g., gender, childbearing status, and religious beliefs) affected patient attitudes about PGT.
Determining the Test to Be Used
Genetic testing is highly specialized. There are also multiple molecular testing methods available, each with its own indications, costs, strengths, and weaknesses. Depending on the method employed and the extent of the analysis, different tests for the same gene will have varying levels of sensitivity and specificity. Even assuming high analytic validity, genetic heterogeneity makes test selection challenging. A number of different genetic syndromes may underlie the development of a particular cancer type. For example, hereditary colorectal cancer may be due to familial adenomatous polyposis (FAP), Lynch syndrome, Peutz-Jeghers syndrome, juvenile polyposis syndrome, or other syndromes. Each of these has a different genetic basis. In addition, different genes may be responsible for the same condition (e.g., Lynch syndrome can be caused by pathogenic variants in one of several mismatch repair [MMR] genes).
In some genes, the same pathogenic variant has been found in multiple, apparently unrelated families. This observation is consistent with a founder effect, wherein a pathogenic variant identified in a contemporary population can be traced back to a small group of founders isolated by geographic, cultural, or other factors. For example, two specific BRCA1 pathogenic variants (68_69delAG and 5266dup, also known in the literature as 185delAG and 5382insC) and one BRCA2 pathogenic variant (5946delT, also known as 6174delT) have been reported to be common in Ashkenazi Jews. Other genes also have reported founder pathogenic variants. The presence of founder pathogenic variants has practical implications for genetic testing. Many laboratories offer directed testing specifically for ethnic-specific alleles. This greatly simplifies the technical aspects of the test but is not without limitations. For example, approximately 15% of BRCA1 and BRCA2 pathogenic variants that occur among Ashkenazim are nonfounder pathogenic variants. Also, for genes in which large genome rearrangements are common in the founder population, ordering additional testing using different techniques may be needed.
Allelic heterogeneity (i.e., different variants within the same gene) can confer different risks or be associated with a different phenotype. For example, though the general rule is that adenomatous polyposis coli (APC) pathogenic variants are associated with hundreds or thousands of colonic polyps and colon cancer of the classical FAP syndrome, some APC pathogenic variants cause a milder clinical picture, with fewer polyps and lower colorectal cancer risk. In addition, other disorders may be part of the FAP spectrum. Pathogenic variants in a certain portion of the APC gene also predispose to retinal changes, for example, when pathogenic variants in a different region of APC predispose to desmoid tumors.
Since hereditary cancer syndromes can have varying presentations and overlapping phenotypes, selecting the appropriate genetic test may require knowledge on the following topics:
- Genetic syndromes.
- Diagnostic methods used for identifying pathogenic variants.
- Correlations between clinical and molecular test findings.
- Information regarding rapidly changing genetic testing options.
See the following PDQ summaries for more information:
- Genetics of Breast and Gynecologic Cancers
- Genetics of Colorectal Cancer
- Genetics of Endocrine and Neuroendocrine Neoplasias
- Genetics of Skin Cancer
- Genetics of Renal Cell Carcinoma
- Genetics of Prostate Cancer
Multigene (panel) testing
Next-generation sequencing (NGS) and the removal of most patent barriers to diagnostic DNA sequencing have resulted in the availability of multigene testing, which can simultaneously test more than 50 genes for pathogenic variants, often at costs comparable to single-gene testing. These multigene panels can include genes with pathogenic variants that are associated with high risks of cancer and genes that confer moderate and uncertain risks. The multigene panels can be limited to specific cancer types (e.g., breast, ovarian, colon) or can include many cancer types. This type of testing has both advantages and disadvantages, and much of the information presented in this section is not based on empirical data but rather on commentaries.
Genetic education and counseling for multigene testing
ASCO has stressed the importance of genetic counseling to ensure patients are adequately informed about the implications of this type of testing and recommends that tests be ordered by cancer genetic professionals. Yet, the use of multigene testing requires modification of traditional approaches to genetic counseling. Optimal evidence-based counseling strategies have not yet been established. Unlike in-person, single-gene pretest genetic counseling models, these approaches have not been examined for outcomes of counseling such as comprehension, satisfaction, psychosocial outcomes, and testing uptake. Table 1 summarizes recommendations from ASCO on elements of pretest genetic counseling and informed consent for germline cancer genetic testing.
| Topic | Traditional Germline Cancer Genetic Testing | Multigene Panel Germline Cancer Genetic Testing |
|---|---|---|
| aAdapted from Robson et al. | ||
| Gene Information | Specific gene(s) or gene variant(s) being tested. | Review of specific genes included in a multigene panel may need to be batched because it is not feasible to individually cover each gene. |
| Risks associated with the gene(s) or gene variants(s) and implications for health care. | Describe high-penetrance gene(s) and/or syndromes included in the multigene panel (i.e., hereditary breast-ovarian syndrome, Lynch syndrome, hereditary diffuse gastric cancer, Li-Fraumeni syndrome), possible detection based on personal and family history and general implications for health care. | |
| Describe generally genes of uncertain clinical utility. | ||
| Possible Test Outcomes | • Pathogenic variant detected. | |
| • No variant detected. | ||
| • Variant of uncertain significance (VUS) detected. | ||
| Variant in a gene for which there is: | ||
| • Limited evidence regarding penetrance. | ||
| • Discordant findings (pathogenic variant identified in a gene that is inconsistent with the patient's personal and/or family history). | ||
| Increased rate of VUS. | ||
| Risks, Benefits, and Limitations of Genetic Testing | Psychosocial implications of test results. | |
| Confidentiality considerations, including privacy, data security, and placement of results (i.e., electronic health record). | ||
| Use of DNA sample(s) for future research. | ||
| Employment and insurance discrimination risks and protections. | ||
| Costs involved in testing and scope of insurance coverage if applicable. | ||
| Whether the genetic health care professional is employed by the testing company. | ||
| Implications of Genetic Testing for Family Members | Pattern of variant transmission and risks of inheritance in children and other family members. | |
| Importance of sharing test results with family members. | ||
| Possible reproductive implications associated with pathogenic variants in genes associated with recessive conditions (i.e., ATM, Fanconi anemia [BRCA2, PALB2], NBN, BLM). | ||
| Use of Genetic Test Results | Implications of genetic test results on health care. | |
Research examining multigene testing
The range of results from NGS multigene panels is emerging in both data from clinical and laboratory series. Several of the studies are collaborations between the two. There are several important caveats about the research that has been conducted so far with regard to multigene testing:
- The studies differ in their aims, approaches, ascertainment of subjects, and panels used.
- Laboratory- and clinic-based studies likely differ with regard to their sampling frames (the population a study draws from and its characteristics). For example, some studies may include testing by a wide variety of health care professionals, some of whom may not be as experienced in triaging, testing, and advising high-risk patients.
- Testing methodologies also differ among laboratories regarding exon/intron coverage, read depth, Sanger sequencing confirmation, and variant interpretation.
- The genes to be tested as part of a multigene panel are constantly changing. In some studies, the composition of multigene panels changed during the course of the study, usually to include more genes.
- Some patient populations included a mix of patients already tested by traditional single-gene methods and those undergoing testing for the first time, making it difficult to establish true diagnostic yield.
- In the studies that replicated previous BRCA testing with a panel, the analytic validity of the NGS multigene panel tests is equivalent to the former single-gene tests, with almost 100% concordance in patients who had both single-gene BRCA testing and multigene testing.
In high-risk individuals who meet criteria for hereditary cancer genetic testing but in whom no pathogenic variant was identified from single-gene testing, panel testing may identify other clinically actionable variants. For example, the additional yield of multigene testing in individuals in whom a BRCA1/BRCA2 pathogenic variant was not detected currently seems to be approximately 4%. The most common non-BRCA pathogenic variants found are in CHEK2, ATM, and PALB2. In some cases, the identification of pathogenic variants from panel testing resulted in additional recommendations for screening and risk reduction beyond what would have been indicated based on family history alone.
Selected reports from 2014 to 2018, which included 1,000 to 10,000 tested individuals, showed variation in pathogenic variant and VUS rates. Pathogenic variant rates ranged from 7% to 14%; VUS rates ranged from 19% to 41% and increased with the number of genes included on the panel, but decreased in the later studies, likely because of larger data pools and refinements in variant interpretation. Additionally, VUS rates were higher in non-White individuals, likely because of the limited availability of test result data needed for accurate determination of risk.
A large study published by a commercial laboratory included more than 252,000 individuals who were tested with a 25-gene panel between 2013 and 2016. The study population did not have prior cancer genetic testing, 97% were female, and 93% met NCCN criteria for hereditary breast and ovarian cancer (HBOC) or Lynch syndrome testing. Half of the pathogenic variants found for HBOC or Lynch syndrome were not in the expected genes associated with these syndromes (BRCA1, BRCA2, MLH1, MSH2, MSH6, and PMS2).
Outcomes of multigene testing
Results from multigene tests have several possible outcomes, including the following:
- No variant detected.
- VUS detected.
- Pathogenic variant in a high-penetrance gene concordant with the existing personal/family history (e.g., a germline MSH2 pathogenic variant in an individual who meets Amsterdam criteria for Lynch syndrome).
- Pathogenic variant in a high-penetrance gene discordant with the existing personal/family history (e.g., a germline CDH1 pathogenic variant in an individual with no personal/family history of gastric cancer).
- Pathogenic variant in a moderate-penetrance gene (e.g., CHEK2, ATM).
- Pathogenic variant in a gene with uncertain cancer risks and/or cancer associations.
Results can also reveal more than one finding given that multiple genes are being tested simultaneously and the elevated rate of VUS. Rates of both VUS and pathogenic variants vary across different racial and ethnic groups.
Considerations when using multigene testing
Using multigene panels can be complex. However, this approach may offer advantages over sequential testing strategies. For example, in some types of cancer, several genes can be associated with specific phenotypes; therefore, testing for all genes associated with a given phenotype can save both time and money. Additionally, multigene panel testing may identify the genetic basis of cancer in families with the following: a differential diagnosis list that includes multiple syndromes, or a family history that does not meet genetic testing criteria for a hereditary cancer syndrome. For more information on factors that make family history difficult to interpret, see the Analysis of the family history section.
However, there can be challenges when employing this testing approach. Clinical laboratories now offer a varying array of clinical cancer susceptibility gene panels. Multigene panels continue to evolve, and the genes included on the panels can change. Other challenges of interpreting multigene test results include higher rates of VUS than those seen in single-gene testing (the rate of VUS increases with the number of genes tested), higher rates of VUS in some racial and ethnic minority populations, and the detection of variants in genes associated with uncertain cancer risks.
Providers who determine a patient's genetic testing strategy may also consider these additional challenges: the patient's out-of-pocket and overall expenses, insurance reimbursement for the genetic test, the genetic test's turn-around-time, ease of ordering the genetic test from a laboratory, the probability of identifying a VUS and managing this finding (via VUS reclassification and access to additional variant data), technical differences between genetic tests (such as the presence of a deletion/duplication assay), patient preference, and a patient's clinical history.
Practice guidelines for optimal clinical use of multigene tests continue to evolve. The NCCN and ASCO guidelines suggest that multigene panel testing may be more efficient when there are multiple cancer syndromes or genes on the differential diagnosis list.
Another important consideration is that multigene tests may include genes in which pathogenic variants are associated with moderate or uncertain penetrance. Management of individuals with pathogenic variants in such genes can present additional challenges, particularly when expert consensus or evidence-based recommendations are not available. For more information on moderate- and low-penetrance, see Figure 1 in Cancer Genetics Overview. Moreover, there may be limited or no evidence to support changes to medical management based on the level of risk or uncertain risk; however, management may still be affected by family history. A framework for clinical management incorporates emerging data on age-specific, lifetime, and absolute cancer risks conferred by pathogenic variants in several moderate-risk genes. For more information about frameworks for clinical management, see the Penetrance of Inherited Susceptibility to Hereditary Breast and/or Gynecologic Cancers section in Genetics of Breast and Gynecologic Cancers.
Regulation of genetic tests
Government regulation of genetic tests to date remains extremely limited in terms of both analytic and clinical validity with little interagency coordination. The Centers for Medicare & Medicaid Services, using the Clinical Laboratory Improvement Act (CLIA), regulates all clinical human laboratory testing performed in the United States for the purposes of generating diagnostic or other health information. CLIA regulations address personnel qualifications, laboratory quality assurance standards, and documentation and validation of tests and procedures. For laboratory tests themselves, CLIA categorizes tests based on the level of complexity into waived tests, moderate complexity, or high complexity. Genetic tests are considered high complexity, which indicates that a high degree of knowledge and skill is required to perform or interpret the test. Laboratories conducting high complexity tests must undergo proficiency testing at specified intervals, which consists of an external review of the laboratory's ability to accurately perform and interpret the test. However, a specialty area specific for molecular and biologic genetic tests has yet to be established; therefore, specific proficiency testing of genetic testing laboratories is not required by CLIA.
In regard to analytic validity, genetic tests fall into two primary categories; test kits and laboratory-developed tests (previously called home brews). Test kits are manufactured for use in laboratories performing the test and include all the reagents necessary to complete the analysis, instructions, performance outcomes, and details about which genetic variants can be detected. The U.S. Food and Drug Administration (FDA) regulates test kits as medical devices; however, despite more than 1,000 available genetic tests, there are fewer than ten FDA-approved test kits. Laboratory-developed tests are performed in a laboratory that assembles its own testing materials in-house; this category represents the most common form of genetic testing. Laboratory-developed tests are subject to the least amount of oversight, as neither CLIA nor the FDA evaluate the laboratories' proficiency in performing the test or clinical validity relative to the accuracy of the test to predict a clinical outcome. The FDA does regulate manufactured analyte-specific reagents (ASRs) as medical devices. These small molecules are used to conduct laboratory-developed tests but can also be made by the laboratory. ASRs made in the laboratory are not subject to FDA oversight. For laboratory-developed tests utilizing manufactured commercially available ASRs, the FDA requires that the test be ordered by a health professional or other individual authorized to order the test by state law. However, this regulation does not distinguish between health providers caring for the patient or health providers who work for the laboratory offering the test.
In addition to the regulation of classical clinical genetic tests is the regulatory oversight of research genetic testing. Laboratories performing genetic testing on a research basis are exempt from CLIA oversight if the laboratory does not report patient-specific results for the diagnosis, prevention, or treatment of any disease or impairment or the assessment of the health of individual patients. However, there are anecdotal reports of research laboratories providing test results for clinical purposes with the caveat that the laboratory recommends that testing be repeated in a clinical CLIA-approved laboratory. In addition, there is no established mechanism that determines when a test has sufficient analytic and clinical validity to be offered clinically. Currently, the decision to offer a genetic test clinically is at the discretion of the laboratory director.
Evidence regarding the implications of this narrow regulatory oversight of genetic tests is limited and consists predominantly of laboratory director responses to quality assurance surveys. A survey of 133 laboratory directors performing genetic tests found that 88% of laboratories employed one or more American Board of Medical Genetics (ABMG)-certified or ABMG-eligible professional geneticists, and 23% had an affiliation with at least one doctoral-prepared geneticist. Eight percent of laboratories did not employ and were not affiliated with doctoral-level genetics professionals. Laboratory-developed tests were performed in 70% of laboratories. Sixty-three percent of laboratories provided an interpretation of the test result as part of the test report. Another survey of 190 laboratory directors found that 97% were CLIA-certified for high complexity testing. Sixteen percent of laboratories reported no specialty area certification; those without specialty certification represented laboratories with the most volume of tests performed and offered the most extensive test selection. Of laboratories with specialty certification, not all had certification relevant to genetic tests, with 48% reporting pathology certification, 46% chemistry certification, and 41% clinical cytogenetics certification. Sixteen percent of directors reported participation in no formal external proficiency testing program, although 77% performed some informal proficiency testing when a formal external proficiency testing program was not available.
The most frequent reason cited for lack of proficiency testing participation was lack of available proficiency testing programs. Laboratory directors estimated that in the past 2 years 37% issued three or fewer incorrect reports, and 35% issued at least four incorrect reports. Analytic errors such as faulty reagent, equipment failure, or human error, increased 40% with each decrease in level of proficiency training completed. An international genetic testing laboratory director survey involving 18 countries found that 64% of the 827 laboratories that responded accepted samples from outside their country. Similar to the U.S. study, 74% reported participation in some form of proficiency testing. Fifty-three percent of the laboratories required a copy of the consent to perform the test, and 72% of laboratories retained specimens indefinitely that were submitted for testing.
The U.S. Department of Health and Human Services Secretary’s Advisory Committee on Genetics, Health, and Society has published a detailed report regarding the adequacy and transparency of the current oversight system for genetic testing in the United States. The Committee identified gaps in the following areas:
- Regulations governing clinical laboratory quality.
- Oversight of the clinical validity of genetic tests.
- The number and identification of laboratories performing genetic tests and the specific genetic tests being performed.
- Level of current knowledge about the clinical usefulness of genetic tests.
- Educational preparation in genetics of health providers, the public health community, patients, and consumers.
In October 2014, the FDA posted the notification regarding its plans to develop draft guidance on the regulation of laboratory-developed tests. Draft guidance documents outlining the framework for regulatory oversight for the industry and clinical laboratories were published later in 2014 for public review and comment. Given the potential of such regulatory action to affect the wide spectrum of genetic tests in clinical practice, proposed draft guidelines have been discussed and reviewed by a number of professional associations, eliciting policy statements and analyses from various professional associations, including the American Society of Human Genetics (ASHG) and the Association for Molecular Pathology. The issue of FDA oversight of laboratory-developed tests remains under consideration.
Direct-to-Consumer (DTC) Genetic Tests
Most genetic testing for cancer and other health risks is offered by health care providers on the basis of a patient’s personal history, family history, or ethnicity. Increasingly, however, individuals can order genetic testing through DTC companies without the input of health care providers. DTC tests may provide information about ancestry, paternity, propensity toward certain physical traits, risk of adverse drug reactions, and disease risks.
Genotyping for carrier status and disease risks
In 2015, the FDA provided clearance for a large DTC company (23andMe) to market carrier screening for Bloom syndrome, which is associated with increased cancer risks in homozygotes as well as other phenotypic features. Subsequently, DTC carrier testing for several conditions became available. In 2017, the FDA allowed 23andMe to market DTC tests for ten diseases or conditions including late-onset Alzheimer disease, Parkinson disease, and hereditary thrombophilia. It is important to note that the carrier and health tests authorized for marketing by the FDA are performed by genotyping, which means that only specific nucleotides or bases are targeted for analysis; sequencing is not performed. Thus, while the false-positive or false-negative rate for a specific genotype is very low (i.e., analytic validity is high), other pathogenic variants are not analyzed, nor is the entire sequence of the gene. Thus, the false-negative rate due to untested pathogenic variants as well as other gene abnormalities is high.
Genotyping for pathogenic variants in BRCA1 and BRCA2
In 2023, the FDA authorized 23andMe to market a test that included 44 pathogenic variants in BRCA1 and BRCA2, including the three BRCA1 and BRCA2 founder pathogenic variants that are commonly identified in individuals of Ashkenazi Jewish descent.
It is crucial for individuals who obtain a BRCA1/BRCA2 (or any health-related) positive result from DTC testing to pursue clinical confirmation of such a result. Clinical confirmation entails repeating the test in a CLIA-certified laboratory, as well as individual review and verification of the result by laboratory personnel.
A potential advantage of DTC testing of these BRCA1/BRCA2 pathogenic variants is that it will identify individuals who would not have been otherwise aware of their increased risk of associated cancers, for example if they have no personal or family history of breast, ovarian, or prostate cancer. This is one of the main arguments for population-based screening for BRCA1/BRCA2 pathogenic variants.
However, a negative result does not rule out other hereditary factors or account for other clinical indicators, genetic and nongenetic, of increased cancer risk. Thus, for most individuals who test negative for the three BRCA1/BRCA2 variants, the results do not provide reassurance about their cancer risks. For high-risk individuals in particular (i.e., those with a history suggestive of hereditary breast/ovarian cancer) a negative result from this limited testing is incomplete, given that it does not assess the presence or absence of other pathogenic variants in BRCA1/BRCA2 or in many other cancer-associated genes.
Consumer-directed clinical testing
Consumer-directed clinical testing is used to describe a hybrid approach to genetic testing, whereupon clinical–grade genetic testing can be initiated and selected by a consumer; however, ordering of the test by an authorized provider (e.g., primary care physician, nurse practitioner, or genetic counselor) is required. The test ordering may be coordinated by the testing company. Genetic counseling may also be offered by the laboratory to explain the results.
With respect to cancer genetic testing, there are clinical, CLIA-certified laboratories that offer multigene (panel) tests as a consumer-directed service. Things to consider when genetic testing is ordered this way include:
- Is the test the same as what would have been ordered by the individual’s own health care provider after review of personal and family history?
Multigene (panel) tests may not include all high- to moderate-risk genes in the differential, or newer/preliminary evidence genes.
- Are variants of uncertain significance (VUS) reported back to the consumer, and if so, is the individual re-contacted if the VUS is reclassified?
This is an important question to ask before testing is performed.
- What is the cost of testing?
Particularly for individuals who meet criteria for testing, insurance may cover the cost, whereas the consumer is responsible for the costs of consumer-directed testing. However, for individuals who do not meet criteria for testing and/or for whom insurance will not pay, the cost of consumer-directed testing may not be higher than out-of-pocket costs when ordered after pretest genetic counseling.
Some insurance companies require patients to have pretest genetic counseling by a credentialed genetics provider (and to meet specific eligibility criteria) in order for the testing to be covered. Consumer-directed testing thus eliminates the need for this requirement.
Testing for single nucleotide variants (SNVs)
In the past, several DTC companies offered only SNV-based testing to generate information about health risks, including risks of cancer. Selection of SNVs may be based on data from genome-wide association studies (GWAS); however, there is no validated algorithm outlining how to generate cancer risk estimates from different SNVs, which individually are generally associated with modestly increased disease risks (usually conferring odds ratios <2.0) or modestly decreased disease risks. For more information, see the GWAS section in Cancer Genetics Overview. As a result, predicted disease risks from different DTC companies may yield different results. For example, a sample comparison of SNV-based risk prediction from two different companies for four different cancers yielded relative risks of 0.64 to 1.42 (excluding the three Ashkenazi BRCA1/BRCA2 founder pathogenic variants). In addition, because commercial companies use different panels of SNVs, there is seldom concordance about the predicted risks for common diseases, and such risk estimates have not been prospectively validated.
Another area of investigation is whether predicted disease risks from SNV testing are consistent with family history–based assessments. Studies using data from one commercial personal genomic testing company revealed that there was generally poor concordance between the SNV and family history risk assessment for common cancers such as breast, prostate, and colon. Importantly, one of these studies highlighted that the majority of individuals with family histories suggestive of hereditary breast/ovarian cancer or Lynch syndrome received SNV results yielding lifetime cancer risks that were average or below average.
Studies have begun to examine whether SNV testing could be used together with other established risk factors to assess the likelihood of developing cancer. For example, adding SNV data to validated breast cancer prediction tools such as those included in the National Cancer Institute's Breast Cancer Risk Assessment Tool (based on the Gail model) may improve the accuracy of risk assessment. However, this approach is not currently FDA-approved.
These findings underscore that SNV testing has not been validated as an accurate risk assessment tool and does not replace the collection, integration, and interpretation of personal and family history risk factor information by qualified health care professionals.
DTC whole-exome/genome sequencing and interpretation
Increasingly, DTC testing companies offer whole-genome sequencing (WGS) or whole-exome sequencing (WES), including SNV data. For more information on WGS and WES, see the Clinical Sequencing section in Cancer Genetics Overview. In addition, consumers who submit their DNA to a DTC lab may have access to their raw sequence data and may consult with other companies, websites, and open-access databases for interpretation. However, these data must be interpreted with caution. A clinical lab found that 40% of variants reported in DTC raw data were false positives (i.e., low analytic validity because the identified variant was not present). In addition, several variants that were designated as increased risk in the raw data were classified as benign by clinical laboratories and public databases. Given the potential for misinterpretation, which may lead to unnecessary medical procedures or testing, these findings underscore the importance of clinical confirmation of all potentially medically actionable gene variants identified by DTC testing.
Some factors to consider when determining the accuracy and utility of sequence data for cancer (or other disease) risk assessment include the sequencing depth of the genes of interest, whether large rearrangements or gene deletions would be detected, and whether or how positive results are confirmed (e.g., through Sanger sequencing). For example, if sequencing depth is low or rare variants cannot be detected, then there is a concern about false-negative results. There is also a risk that sequence changes will be erroneously labeled as pathogenic when confirmatory testing or different interpretative approaches would determine that the variant identified is benign (false positive). When WES or WGS is performed, VUS are also likely to be identified, and DTC companies have varying protocols for classification, which may or may not be consistent with national guidelines. In addition, as evidence evolves and variants are reclassified, consumers need to be aware of the process the DTC lab has, if any, for updating information and re-contacting consumers with revised interpretations.
Considerations
There may be potential benefits associated with DTC testing. DTC marketing and provision of genetic tests may promote patient autonomy. Individuals may develop an increased awareness of the importance of family history, the relationship between risk and family history, the role of genetics in disease, and a better understanding of the value of genetic counseling. Although results of SNV-based DTC testing appear to motivate some individuals to seek the advice of their doctor, make lifestyle changes, and pursue screening tests, short-term modest effects on risk perception after notification of an elevated risk (e.g., for cancer) may not significantly alter lifestyle or cancer screening behaviors. Further, psychological distress has not been widely reported among consumers who have undergone DTC testing for a variety of conditions. However, little is known about how individuals respond after learning that they carry pathogenic variants in high-risk genes such as BRCA1/BRCA2 when testing is performed within a DTC context and without traditional forms of pre- and posttest genetic education and counseling.
Given the complexity of genomic testing, several professional organizations have released position statements about DTC genetic testing. For example, in 2010, ASCO published a position statement outlining several considerations related to DTC cancer genomic tests, including those mentioned above. They endorsed pre- and posttest genetic counseling and informed consent by qualified health care professionals. ASCO’s 2015 position statement on genetic and genomic testing for cancer susceptibility reinforces the importance of provider education given the complexity of genomic testing and interpretation and discusses their recommendations for regulatory review of genomic tests, including those offered by DTC companies.
In 2016, a statement by the American College of Medical Genetics and Genomics about DTC genetic testing similarly endorsed the involvement of qualified genetics professionals in the processes of test ordering and interpretation. The statement also emphasized the need to incorporate established methods of risk assessment into disease risk prediction (such as personal and family medical history information) and stressed that consumers need to be informed about the potential limitations and risks associated with DTC testing.
Informed Consent
Informed consent can enhance preparedness for testing, including careful weighing of benefits and limitations of testing, minimization of adverse psychosocial outcomes, appropriate use of medical options, and a strengthened provider-patient relationship based on honesty, support, and trust.
Consensus exists among experts that a process of informed consent should be an integral part of the pretest counseling process. This view is driven by several ethical dilemmas that can arise in genetic susceptibility testing. The most commonly cited concern is the possibility of insurance or employment discrimination if a test result, or even the fact that an individual has sought or is seeking testing, is disclosed. In 2008, Congress passed the Genetic Information Nondiscrimination Act of 2008 (GINA). This federal law provides protections related to health insurance and employment discrimination based on genetic information. However, GINA does not cover life, disability, or long-term-care insurance discrimination. For more information, see the GINA section. A related issue involves stigmatization that may occur when an individual who may never develop the condition in question, or may not do so for decades, receives genetic information and is labeled or labels himself or herself as ill. Finally, in the case of genetic testing, medical information given to one individual has immediate implications for biologic relatives. These implications include not only the medical risks but also disruptions in familial relationships. The possibility for coercion exists when one family member wants to be tested but, to do so optimally, must first obtain genetic material or information from other family members.
Inclusion of an informed consent process in counseling can facilitate patient autonomy. It may also reduce the potential for misunderstanding between patient and provider. Many clinical programs provide opportunities for individuals to review their informed consent during the genetic testing and counseling process. Initial informed consent provides a verbal and/or written overview of the process.
Some programs use a second informed consent process prior to disclosure to the individual of his or her genetic test results. This process allows for the possibility that a person may change his or her mind about receiving test results. After the test result has been disclosed, a third informed consent discussion often occurs. This discussion concerns issues regarding sharing the genetic test result with health providers and/or interested family members, currently or in the future. Obtaining written permission to provide the test result to others in the family who are at risk can avoid vexing problems in the future should the individual not be available to release his or her results.
Core elements of informed consent
Major elements of an informed consent discussion are highlighted in the preceding discussion. The critical elements, as described in the literature, include the following:
- Specific test being performed.
- Elicitation and discussion of a person’s expectations, beliefs, goals, and motivations.
- Explanation of how inheritance of genetic factors may affect cancer susceptibility.
- Clarification of a person’s increased risk status.
- Discussion of potential benefits, risks, and limitations of testing.
- Discussion of costs and logistics of testing and follow-up.
- Discussion of possible outcomes of testing (e.g., true positive, true negative, VUS, inconclusive, false positive, false negative, secondary findings).
- Discussion of medical management options based on risk assessment and/or test results available for those who choose to test, for those who choose not to test, and for those who have positive, negative, or inconclusive results.
- Data on efficacy of methods of cancer prevention and early detection.
- Discussion of possible psychological, social, economic, and family dynamic ramifications of testing or not testing.
- Discussion of a genetic test result's implications for family members; patients may be asked to inform their family member(s) about their genetic test results.
- Discussion of alternatives to genetic testing (e.g., tissue banking, risk assessment without genetic testing).
- Attainment of verbal and written informed consent or clarification of the decision to decline testing.
All individuals considering genetic testing should be informed that they have several options even after the genetic testing has been completed. They may decide to receive the results at the posttest meeting, delay result notification, or less commonly, not receive the results of testing. They should be informed that their interest in receiving results will be addressed at the beginning of the posttest meeting and that time will be available to review their concerns and thoughts on notification. It is important that individuals receive this information during the pretest counseling to ensure added comfort with the decision to decline or defer result notification even when test results become available.
Testing in children
Genetic testing for pathogenic variants in cancer susceptibility genes in children is particularly complex. While both parents and providers may request or recommend testing for minor children, many experts recommend that unless there is evidence that the test result will influence the medical management of the child or adolescent, genetic testing should be deferred until legal adulthood (age 18 y or older) because of concerns about autonomy, potential discrimination, and possible psychosocial effects. A number of cancer syndromes include childhood disease risk, such as retinoblastoma, multiple endocrine neoplasia (MEN) types 1 and 2 (MEN1 and MEN2), neurofibromatosis types 1 and 2 (NF1 and NF2), Beckwith–Wiedemann syndrome, Fanconi anemia, FAP, and Von Hippel-Lindau disease (VHL). As a consequence, decisions about genetic testing in children are made in the context of a specific gene in which a pathogenic variant is suspected. The ASCO statement on genetic testing for cancer susceptibility maintains that the decision to consider offering childhood genetic testing should take into account not only the risk of childhood malignancy but also the evidence associated with risk reduction interventions for that disorder. Specifically, ASCO recommends that:
- When screening or preventive strategies during childhood are available (e.g., MEN and FAP), testing should be encouraged on clinical grounds.
- When no risk reduction strategies are available in childhood and the probability of developing a malignancy during childhood is very low (e.g., hereditary breast/ovarian cancer syndrome), testing should not be offered.
- Some patients may be at risk of developing a malignancy during childhood without the availability of validated risk-reduction strategies (e.g., TP53 pathogenic variants). The decision to test in such circumstances is particularly controversial.
Special considerations are required when genetic counseling and testing for pathogenic variants in cancer susceptibility genes are considered in children. The first issue is the age of the child. Young children, especially those younger than 10 years, may not be involved or may have limited involvement in the decision to be tested, and some may not participate in the genetic counseling process. In these cases, the child’s parents or other legal surrogate will be involved in the genetic counseling and will ultimately be responsible for making the decision to proceed with testing. Counseling under these circumstances incorporates a discussion of how test results will be shared with the child when he or she is older. Children aged 10 to 17 years may have more involvement in the decision-making process. In a qualitative study of parents and children aged 10 to 17 years assessing decision making for genetic research participation, older, more mature children and families with open communication styles were more likely to have joint decision making. The majority of children in this study felt that they should have the right to make the final decision for genetic research participation, although many would seek input from their parents. While this study is specific to genetic research participation, the findings allude to the importance children aged 10 to 17 years place on personal decision making regarding factors that impact them. Unfortunately cognitive and psychosocial development may not consistently correlate with the age of the child. Therefore, careful assessment of the child’s developmental stage may help in the genetic counseling and testing process to facilitate parent and child adaptation to the test results. Another complicating factor includes potential risks for discrimination. For more information, see the Employment and Insurance Discrimination section.
The consequences of genetic testing in children have been reviewed. In contrast to observations in adults, young children in particular are vulnerable to changes in parent and child bonding based on test results. Genetic testing could interfere with the development of self-concept and self-esteem. Children may also be at risk of developing feelings of survivor guilt or heightened anxiety. All children are especially susceptible to not understanding the testing, results, or implications for their health. As children mature, they begin to have decreased dependency on their parents while developing their personal identity. This can be altered in the setting of a serious health condition or an inherited disorder. Older children are beginning to mature physically and develop intimate relationships while also changing their idealized view of their parents. All of this can be influenced by the results of a genetic test. In its recommendations for genetic testing in asymptomatic minors, the European Society of Human Genetics emphasizes that parents have a responsibility to inform their children about their genetic risk and to communicate this information in a way that is tailored to the child’s age and developmental level.
In summary, genetic testing may be considered in children if the results may alter the child's medical treatment and positively impact his/her health outcomes. Deferral of genetic testing is suggested when the risk of childhood malignancy is low or absent and/or there is no evidence that interventions can reduce risk. When genetic testing is offered to a child, the child’s developmental stage is used to determine his/her involvement and who has legal authority to provide consent. It is important for providers to understand a family's psychosocial state and dynamics regarding potential genetic test results; this will enable the provider to give appropriate support when disclosing the child's genetic test result.
For more information about testing children for hereditary cancer syndromes, see the following PDQ summaries:
- Genetics of Breast and Gynecologic Cancers
- Genetics of Colorectal Cancer
- Genetics of Endocrine and Neuroendocrine Neoplasias
Testing in vulnerable populations
Genetic counseling and testing require special considerations when used in vulnerable populations. In 1995, the American Society of Human Genetics published a position statement on the ethical, legal, and psychosocial implications of genetic testing in children and adolescents as a vulnerable population. However, vulnerable populations encompass more than just children. Federal policy applicable to research involving human subjects, 45 CFR Code of Federal Regulations part 46 Protection Of Human Subjects, considers the following groups as potentially vulnerable populations: prisoners, traumatized and comatose patients, terminally ill patients, elderly/aged individuals who are cognitively impaired and/or institutionalized, minorities, students, employees, and individuals from outside the United States. Specific to genetic testing, the International Society of Nurses in Genetics further expanded the definition of vulnerable populations to also include individuals with hearing and language deficits or conditions limiting communication (for example, language differences and concerns with reliable translation), cognitive impairment, psychiatric disturbances, clients undergoing stress due to a family situation, those without financial resources, clients with acute or chronic illness and in end-of-life, and those in whom medication may impair reasoning.
Genetic counseling and testing in vulnerable populations raises special considerations. The aim of genetic counseling is to help people understand and adapt to the medical, psychological, and familial implications of genetic contributions to disease, which in part involves the meaningful exchange of factual information. In a vulnerable population, health care providers need to be sensitive to factors that can impact the ability of the individual to comprehend the information. In particular, in circumstances of cognitive impairment or intellectual disability, special attention is paid to whether the individual’s legally authorized representative should be involved in the counseling, informed consent, and testing process.
Providers need to assess all patients for their ability to make an uncoerced, autonomous, informed decision prior to proceeding with genetic testing. Populations that do not seem vulnerable (e.g., legally adult college students) may actually be deemed vulnerable because of undue coercion for testing by their parents or the threat of withholding financial support by their parents based on a testing decision inconsistent with the parent’s wishes. Alteration of the genetic counseling and testing process may be necessary depending on the situation, such as counseling and testing in terminally ill individuals who opt for testing for the benefit of their children, but given their impending death, results may have no impact on their own health care or may not be available before their death. In summary, genetic counseling and testing requires that the health care provider assess all individuals for any evidence of vulnerability, and if present, be sensitive to those issues, modify genetic counseling based on the specific circumstances, and avoid causing additional harm.
References
- Robson ME, Storm CD, Weitzel J, et al.: American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol 28 (5): 893-901, 2010.
- Robson ME, Bradbury AR, Arun B, et al.: American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. J Clin Oncol 33 (31): 3660-7, 2015.
- Gustafson SL, Raymond VM, Marvin ML, et al.: Outcomes of genetic evaluation for hereditary cancer syndromes in unaffected individuals. Fam Cancer 14 (1): 167-74, 2015.
- Berliner JL, Cummings SA, Boldt Burnett B, et al.: Risk assessment and genetic counseling for hereditary breast and ovarian cancer syndromes-Practice resource of the National Society of Genetic Counselors. J Genet Couns 30 (2): 342-360, 2021.
- Clain E, Trosman JR, Douglas MP, et al.: Availability and payer coverage of BRCA1/2 tests and gene panels. Nat Biotechnol 33 (9): 900-2, 2015.
- Walcott FL, Dunn BK: Legislation in the genomic era: the Affordable Care Act and genetic testing for cancer risk assessment. Genet Med 17 (12): 962-4, 2015.
- The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs - Set 12. Baltimore, Md: Centers for Medicare & Medicaid Services, 2013. Available online. Last accessed January 17, 2024.
- Facing Our Risk of Cancer Empowered (FORCE): Paying for Genetic Services. Tampa, FL: FORCE, 2016. Available online. Last accessed January 17, 2024.
- Offit K, Kohut K, Clagett B, et al.: Cancer genetic testing and assisted reproduction. J Clin Oncol 24 (29): 4775-82, 2006.
- Offit K, Sagi M, Hurley K: Preimplantation genetic diagnosis for cancer syndromes: a new challenge for preventive medicine. JAMA 296 (22): 2727-30, 2006.
- Wang CW, Hui EC: Ethical, legal and social implications of prenatal and preimplantation genetic testing for cancer susceptibility. Reprod Biomed Online 19 (Suppl 2): 23-33, 2009.
- Meyer S, Tischkowitz M, Chandler K, et al.: Fanconi anaemia, BRCA2 mutations and childhood cancer: a developmental perspective from clinical and epidemiological observations with implications for genetic counselling. J Med Genet 51 (2): 71-5, 2014.
- Sawyer SL, Tian L, Kähkönen M, et al.: Biallelic mutations in BRCA1 cause a new Fanconi anemia subtype. Cancer Discov 5 (2): 135-42, 2015.
- Rich TA, Liu M, Etzel CJ, et al.: Comparison of attitudes regarding preimplantation genetic diagnosis among patients with hereditary cancer syndromes. Fam Cancer 13 (2): 291-9, 2014.
- Frank TS, Deffenbaugh AM, Reid JE, et al.: Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol 20 (6): 1480-90, 2002.
- Nieuwenhuis MH, Vasen HF: Correlations between mutation site in APC and phenotype of familial adenomatous polyposis (FAP): a review of the literature. Crit Rev Oncol Hematol 61 (2): 153-61, 2007.
- Knudsen AL, Bülow S, Tomlinson I, et al.: Attenuated familial adenomatous polyposis: results from an international collaborative study. Colorectal Dis 12 (10 Online): e243-9, 2010.
- Offit K, Bradbury A, Storm C, et al.: Gene patents and personalized cancer care: impact of the Myriad case on clinical oncology. J Clin Oncol 31 (21): 2743-8, 2013.
- Robson M: Multigene panel testing: planning the next generation of research studies in clinical cancer genetics. J Clin Oncol 32 (19): 1987-9, 2014.
- Domchek SM, Bradbury A, Garber JE, et al.: Multiplex genetic testing for cancer susceptibility: out on the high wire without a net? J Clin Oncol 31 (10): 1267-70, 2013.
- Hiraki S, Rinella ES, Schnabel F, et al.: Cancer risk assessment using genetic panel testing: considerations for clinical application. J Genet Couns 23 (4): 604-17, 2014.
- Cragun D, Radford C, Dolinsky JS, et al.: Panel-based testing for inherited colorectal cancer: a descriptive study of clinical testing performed by a US laboratory. Clin Genet 86 (6): 510-20, 2014.
- Couch FJ, Hart SN, Sharma P, et al.: Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol 33 (4): 304-11, 2015.
- LaDuca H, Stuenkel AJ, Dolinsky JS, et al.: Utilization of multigene panels in hereditary cancer predisposition testing: analysis of more than 2,000 patients. Genet Med 16 (11): 830-7, 2014.
- Kurian AW, Hare EE, Mills MA, et al.: Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol 32 (19): 2001-9, 2014.
- Tung N, Battelli C, Allen B, et al.: Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer 121 (1): 25-33, 2015.
- Moran O, Nikitina D, Royer R, et al.: Revisiting breast cancer patients who previously tested negative for BRCA mutations using a 12-gene panel. Breast Cancer Res Treat 161 (1): 135-142, 2017.
- Frey MK, Kim SH, Bassett RY, et al.: Rescreening for genetic mutations using multi-gene panel testing in patients who previously underwent non-informative genetic screening. Gynecol Oncol 139 (2): 211-5, 2015.
- Lincoln SE, Kobayashi Y, Anderson MJ, et al.: A Systematic Comparison of Traditional and Multigene Panel Testing for Hereditary Breast and Ovarian Cancer Genes in More Than 1000 Patients. J Mol Diagn 17 (5): 533-44, 2015.
- Desmond A, Kurian AW, Gabree M, et al.: Clinical Actionability of Multigene Panel Testing for Hereditary Breast and Ovarian Cancer Risk Assessment. JAMA Oncol 1 (7): 943-51, 2015.
- Kapoor NS, Curcio LD, Blakemore CA, et al.: Multigene Panel Testing Detects Equal Rates of Pathogenic BRCA1/2 Mutations and has a Higher Diagnostic Yield Compared to Limited BRCA1/2 Analysis Alone in Patients at Risk for Hereditary Breast Cancer. Ann Surg Oncol 22 (10): 3282-8, 2015.
- Ricker C, Culver JO, Lowstuter K, et al.: Increased yield of actionable mutations using multi-gene panels to assess hereditary cancer susceptibility in an ethnically diverse clinical cohort. Cancer Genet 209 (4): 130-7, 2016.
- Hermel DJ, McKinnon WC, Wood ME, et al.: Multi-gene panel testing for hereditary cancer susceptibility in a rural Familial Cancer Program. Fam Cancer 16 (1): 159-166, 2017.
- Eliade M, Skrzypski J, Baurand A, et al.: The transfer of multigene panel testing for hereditary breast and ovarian cancer to healthcare: What are the implications for the management of patients and families? Oncotarget 8 (2): 1957-1971, 2017.
- Yurgelun MB, Allen B, Kaldate RR, et al.: Identification of a Variety of Mutations in Cancer Predisposition Genes in Patients With Suspected Lynch Syndrome. Gastroenterology 149 (3): 604-13.e20, 2015.
- Susswein LR, Marshall ML, Nusbaum R, et al.: Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genet Med 18 (8): 823-32, 2016.
- Shirts BH, Casadei S, Jacobson AL, et al.: Improving performance of multigene panels for genomic analysis of cancer predisposition. Genet Med 18 (10): 974-81, 2016.
- Caswell-Jin JL, Gupta T, Hall E, et al.: Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med 20 (2): 234-239, 2018.
- Rosenthal ET, Bernhisel R, Brown K, et al.: Clinical testing with a panel of 25 genes associated with increased cancer risk results in a significant increase in clinically significant findings across a broad range of cancer histories. Cancer Genet 218-219: 58-68, 2017.
- Roberts ME, Susswein LR, Janice Cheng W, et al.: Ancestry-specific hereditary cancer panel yields: Moving toward more personalized risk assessment. J Genet Couns 29 (4): 598-606, 2020.
- Fecteau H, Vogel KJ, Hanson K, et al.: The evolution of cancer risk assessment in the era of next generation sequencing. J Genet Couns 23 (4): 633-9, 2014.
- Hall MJ, Forman AD, Pilarski R, et al.: Gene panel testing for inherited cancer risk. J Natl Compr Canc Netw 12 (9): 1339-46, 2014.
- Easton DF, Pharoah PD, Antoniou AC, et al.: Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med 372 (23): 2243-57, 2015.
- Eggington JM, Bowles KR, Moyes K, et al.: A comprehensive laboratory-based program for classification of variants of uncertain significance in hereditary cancer genes. Clin Genet 86 (3): 229-37, 2014.
- Wolfe Schneider K, Anguiano A, Axell L, et al.: Collaboration of colorado cancer genetic counselors to integrate next generation sequencing panels into clinical practice. J Genet Couns 23 (4): 640-6, 2014.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 2.2024. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023. Available online with free registration. Last accessed October 31, 2023.
- Tung N, Domchek SM, Stadler Z, et al.: Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol 13 (9): 581-8, 2016.
- Hudson KL, Murphy JA, Kaufman DJ, et al.: Oversight of US genetic testing laboratories. Nat Biotechnol 24 (9): 1083-90, 2006.
- Schwartz MK: Genetic testing and the clinical laboratory improvement amendments of 1988: present and future. Clin Chem 45 (5): 739-45, 1999.
- Javitt GH, Hudson K: Federal neglect: regulation of genetic testing. Issues Sci Technol 22: 58-66, 2006. Also available online. Last accessed January 17, 2024.
- McGovern MM, Benach M, Wallenstein S, et al.: Personnel standards and quality assurance practices of biochemical genetic testing laboratories in the United States. Arch Pathol Lab Med 127 (1): 71-6, 2003.
- McGovern MM, Elles R, Beretta I, et al.: Report of an international survey of molecular genetic testing laboratories. Community Genet 10 (3): 123-31, 2007.
- Ferreira-Gonzalez A, Teutsch S, Williams MS, et al.: US system of oversight for genetic testing: a report from the Secretary's Advisory Committee on Genetics, Health and Society. Per Med 5 (5): 521-528, 2008.
- Food and Drug Administration: Notification to Congress: FDA’s Laboratory Developed Tests Framework. Silver Spring, Md: Food and Drug Administration, 2014. Available online. Last accessed January 17, 2024.
- U.S. Food and Drug Administration: FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions. Silver Spring, Md: U.S. Food and Drug Administration, 2017. Available online. Last accessed January 17, 2024.
- Wanner M: Genomes Versus Exomes Versus Genotypes. Bar Harbor, Me: The Jackson Library, 2016. Available online. Last accessed January 17, 2024.
- U.S. Food and Drug Administration: FDA News Release: FDA Roundup (September 1, 2023). 2023. Available online. Last accessed February 26, 2024.
- U.S. Food and Drug Administration: FDA authorizes, with special controls, direct-to-consumer test that reports three mutations in the BRCA breast cancer genes. Silver Spring, Md: U.S. Food and Drug Administration, 2018. Available online. Last accessed January 17, 2024.
- Ramos E, Weissman SM: The dawn of consumer-directed testing. Am J Med Genet C Semin Med Genet 178 (1): 89-97, 2018.
- Couch FJ, Nathanson KL, Offit K: Two decades after BRCA: setting paradigms in personalized cancer care and prevention. Science 343 (6178): 1466-70, 2014.
- Bellcross CA, Page PZ, Meaney-Delman D: Direct-to-consumer personal genome testing and cancer risk prediction. Cancer J 18 (4): 293-302, 2012 Jul-Aug.
- Swan M: Multigenic condition risk assessment in direct-to-consumer genomic services. Genet Med 12 (5): 279-88, 2010.
- Kalf RR, Mihaescu R, Kundu S, et al.: Variations in predicted risks in personal genome testing for common complex diseases. Genet Med 16 (1): 85-91, 2014.
- Aiyar L, Shuman C, Hayeems R, et al.: Risk estimates for complex disorders: comparing personal genome testing and family history. Genet Med 16 (3): 231-7, 2014.
- Heald B, Edelman E, Eng C: Prospective comparison of family medical history with personal genome screening for risk assessment of common cancers. Eur J Hum Genet 20 (5): 547-51, 2012.
- Bloss CS, Topol EJ, Schork NJ: Association of direct-to-consumer genome-wide disease risk estimates and self-reported disease. Genet Epidemiol 36 (1): 66-70, 2012.
- Gail MH, Brinton LA, Byar DP, et al.: Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 81 (24): 1879-86, 1989.
- McCarthy AM, Armstrong K, Handorf E, et al.: Incremental impact of breast cancer SNP panel on risk classification in a screening population of white and African American women. Breast Cancer Res Treat 138 (3): 889-98, 2013.
- Mealiffe ME, Stokowski RP, Rhees BK, et al.: Assessment of clinical validity of a breast cancer risk model combining genetic and clinical information. J Natl Cancer Inst 102 (21): 1618-27, 2010.
- Glusman G, Cariaso M, Jimenez R, et al.: Low budget analysis of Direct-To-Consumer genomic testing familial data. F1000Res 1: 3, 2012.
- Cariaso M, Lennon G: SNPedia: a wiki supporting personal genome annotation, interpretation and analysis. Nucleic Acids Res 40 (Database issue): D1308-12, 2012.
- Tandy-Connor S, Guiltinan J, Krempely K, et al.: False-positive results released by direct-to-consumer genetic tests highlight the importance of clinical confirmation testing for appropriate patient care. Genet Med 20 (12): 1515-1521, 2018.
- Berg JS, Khoury MJ, Evans JP: Deploying whole genome sequencing in clinical practice and public health: meeting the challenge one bin at a time. Genet Med 13 (6): 499-504, 2011.
- Richards S, Aziz N, Bale S, et al.: Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17 (5): 405-24, 2015.
- McCabe LL, McCabe ER: Direct-to-consumer genetic testing: access and marketing. Genet Med 6 (1): 58-9, 2004 Jan-Feb.
- Bansback N, Sizto S, Guh D, et al.: The effect of direct-to-consumer genetic tests on anticipated affect and health-seeking behaviors: a pilot survey. Genet Test Mol Biomarkers 16 (10): 1165-71, 2012.
- Kaufman DJ, Bollinger JM, Dvoskin RL, et al.: Risky business: risk perception and the use of medical services among customers of DTC personal genetic testing. J Genet Couns 21 (3): 413-22, 2012.
- Bloss CS, Schork NJ, Topol EJ: Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med 364 (6): 524-34, 2011.
- van der Wouden CH, Carere DA, Maitland-van der Zee AH, et al.: Consumer Perceptions of Interactions With Primary Care Providers After Direct-to-Consumer Personal Genomic Testing. Ann Intern Med 164 (8): 513-22, 2016.
- Carere DA, VanderWeele T, Moreno TA, et al.: The impact of direct-to-consumer personal genomic testing on perceived risk of breast, prostate, colorectal, and lung cancer: findings from the PGen study. BMC Med Genomics 8: 63, 2015.
- Gray SW, Gollust SE, Carere DA, et al.: Personal Genomic Testing for Cancer Risk: Results From the Impact of Personal Genomics Study. J Clin Oncol 35 (6): 636-644, 2017.
- ACMG Board of Directors: Direct-to-consumer genetic testing: a revised position statement of the American College of Medical Genetics and Genomics. Genet Med 18 (2): 207-8, 2016.
- Geller G, Botkin JR, Green MJ, et al.: Genetic testing for susceptibility to adult-onset cancer. The process and content of informed consent. JAMA 277 (18): 1467-74, 1997.
- Hudson KL, Holohan MK, Collins FS: Keeping pace with the times--the Genetic Information Nondiscrimination Act of 2008. N Engl J Med 358 (25): 2661-3, 2008.
- Geller G, Doksum T, Bernhardt BA, et al.: Participation in breast cancer susceptibility testing protocols: influence of recruitment source, altruism, and family involvement on women's decisions. Cancer Epidemiol Biomarkers Prev 8 (4 Pt 2): 377-83, 1999.
- American College of Medical Genetics: Genetic susceptibility to breast and ovarian cancer: assessment, counseling and testing guidelines. New York State Department of Health, American College of Medical Genetics Foundation, 1999.
- McKinnon WC, Baty BJ, Bennett RL, et al.: Predisposition genetic testing for late-onset disorders in adults. A position paper of the National Society of Genetic Counselors. JAMA 278 (15): 1217-20, 1997.
- Phillips A, Niemiec E, Howard HC, et al.: Communicating genetic information to family members: analysis of consent forms for diagnostic genomic sequencing. Eur J Hum Genet 28 (9): 1160-1167, 2020.
- Bradbury AR, Patrick-Miller L, Egleston B, et al.: Parent opinions regarding the genetic testing of minors for BRCA1/2. J Clin Oncol 28 (21): 3498-505, 2010.
- O'Neill SC, Peshkin BN, Luta G, et al.: Primary care providers' willingness to recommend BRCA1/2 testing to adolescents. Fam Cancer 9 (1): 43-50, 2010.
- Nelson RM, Botkjin JR, Kodish ED, et al.: Ethical issues with genetic testing in pediatrics. Pediatrics 107 (6): 1451-5, 2001.
- Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. American Society of Human Genetics Board of Directors, American College of Medical Genetics Board of Directors. Am J Hum Genet 57 (5): 1233-41, 1995.
- Wertz DC, Fanos JH, Reilly PR: Genetic testing for children and adolescents. Who decides? JAMA 272 (11): 875-81, 1994.
- Field M, Shanley S, Kirk J: Inherited cancer susceptibility syndromes in paediatric practice. J Paediatr Child Health 43 (4): 219-29, 2007.
- Tischkowitz M, Rosser E: Inherited cancer in children: practical/ethical problems and challenges. Eur J Cancer 40 (16): 2459-70, 2004.
- Fanos JH: Developmental tasks of childhood and adolescence: implications for genetic testing. Am J Med Genet 71 (1): 22-8, 1997.
- Bernhardt BA, Tambor ES, Fraser G, et al.: Parents' and children's attitudes toward the enrollment of minors in genetic susceptibility research: implications for informed consent. Am J Med Genet A 116 (4): 315-23, 2003.
- European Society of Human Genetics: Genetic testing in asymptomatic minors: Recommendations of the European Society of Human Genetics. Eur J Hum Genet 17 (6): 720-1, 2009.
- Borry P, Evers-Kiebooms G, Cornel MC, et al.: Genetic testing in asymptomatic minors: background considerations towards ESHG Recommendations. Eur J Hum Genet 17 (6): 711-9, 2009.
- Resta R, Biesecker BB, Bennett RL, et al.: A new definition of Genetic Counseling: National Society of Genetic Counselors' Task Force report. J Genet Couns 15 (2): 77-83, 2006.
Risk Communication
Specific clinical programs for risk management may be offered to individuals with an increased genetic risk of cancer. These programs may differ from those offered to individuals of average risk in several ways: screening may be initiated at an earlier age or involve shorter screening intervals; screening strategies not in routine use, such as screening for ovarian cancer, may be offered; and interventions to reduce cancer risk, such as risk-reducing surgery, may be offered. Current recommendations are summarized in the PDQ summaries addressing the genetics of specific cancers.
The goal of genetic education and counseling is to help individuals understand their personal risk status, recognize their options for cancer risk management, and explore their feelings regarding their personal risk status. Counseling focuses on obtaining and giving information, promoting autonomous decision making, and facilitating informed consent if genetic testing is pursued.
Optimally, education and counseling about cancer risk includes providing the following information:
- Purpose, strengths, and limitations of cancer risk assessment.
- Basic genetics and patterns of inheritance.
- Genetic basis of cancer.
- Clinical features of relevant hereditary cancer syndromes.
- Evidence of a hereditary cancer syndrome from the consultand's personal and family history.
- Options for clarifying cancer risk, including genetic testing, if indicated.
- Options available for risk management, including data (or lack of data) on the efficacy of different measures for early detection and risk reduction.
- Signs and symptoms of cancer.
When a clinically valid genetic test is available, education and counseling for genetic testing typically includes the following:
- Risk of having a pathogenic variant and patterns of transmission.
- Alternatives to genetic testing.
- Risks, benefits, and limitations of genetic testing, including psychological and discriminatory risks.
- Possible test outcomes, including likelihood of uninformative results and identifying variants of uncertain significance.
- Sensitivity of the genetic test, including the techniques utilized to perform the test and their associated limitations.
- Health care management options based on possible test results.
- Implications for children and other family members based on pattern of transmission.
- Dissemination of risk and genetic information to family members.
- Cost associated with testing, counseling, medical management, and options for insurance coverage.
- How genetic information and genetic test results will be recorded in the medical record.
- Specimen storage and reuse, if applicable.
If a second session is held to disclose and interpret genetic test results, education and counseling focuses on the following:
- Interpretation of test results.
- Discussion of further testing that may clarify risk (e.g., large rearrangement testing and testing the other genes based on the patient's differential cancer syndrome list).
- Assessment of the emotional and behavioral responses to genetic test results.
- Recommendations for coping and communication strategies to address issues related to cancer risk.
- Cancer risk management recommendations.
- Risk analysis and dissemination of risk results to family members.
The process of counseling may require more than one visit to address medical, genetic testing, and psychosocial support issues. Additional case-related preparation time is spent before and after the consultation sessions to obtain and review medical records, complete case documentation, seek information about differential diagnoses, identify appropriate laboratories for genetic tests, find patient support groups, research resources, and communicate with or refer to other specialists.
Information about inherited risk of cancer is growing rapidly. Many of the issues discussed in a counseling session may need to be revisited as new information emerges. At the end of the counseling process, individuals are typically reminded of the possibility that future research may provide new options and/or new information on risk. Individuals may be advised to check in with the health care provider periodically to determine whether new information is sufficient to merit an additional counseling session. The obligation of health care providers to recontact individuals when new genetic testing or treatment options are available is controversial, and standards have not been established.
Methods of Risk Presentation
Using probability to communicate risk may overestimate risk certainty; this is especially true when risk estimates have wide confidence intervals or when the patient has characteristics that differ greatly from that of the sample that the risk estimate was based on. Finally, there are wide variations in individuals’ level of understanding of mathematical concepts (i.e., numeracy). For all the above reasons, conveying risk in multiple ways, both numerically and verbally, with discussion of important caveats, may be a useful strategy to increase risk comprehension. The numerical format that facilitates the best understanding is natural frequencies because frequencies include information concerning the denominator, the reference group to which the individual may refer. In general, logarithmic scales are to be avoided. Additionally, important “contextual” risks may be included with the frequency in order to increase risk comprehension; these may include how the person’s risk compares with those who do not have the risk factor in question and the risks associated with common hazards, such as being in a car accident. Additional suggestions include being consistent in risk formats (do not mix odds and percentages), using the same denominator across risk estimates, avoiding decimal points, including base rate information, and providing more explanation if the risk is less than 1%.
The communication of risk may be numerical or visual. Use of multiple strategies may increase comprehension and retention of cancer genetic risk information. Recently, use of visual risk communication strategies has increased (e.g., histograms, pie charts, and Venn diagrams). Visual depictions of risk may be very useful when working with visual learners, but research that confirms this is lacking. A study published in 2008 examined the use of two different visual aids to communicate breast cancer risk. Women at an increased risk of breast cancer were randomized to receive feedback via a bar graph alone or a bar graph plus a frequency diagram (i.e., highlighted human figures). Overall results indicated that there were no differences in improved accuracy of risk perception between the two groups. However, there was a greater improvement in accuracy of risk perception among the group of women who inaccurately perceived very high risk at baseline and received both visual aids.
Communication Strategies
Studies have examined novel channels to communicate genetic cancer risk information, deliver psychosocial support, and standardize the genetic counseling process for individuals at increased risk of cancer. Much of this literature has attempted to make the genetic counseling session more efficient or to limit the need for the counselor to address basic genetic principles in the session to free up time for the client’s personal and emotional concerns about his or her risk. For example, the receipt of genetic feedback for BRCA1/BRCA2 and mismatch repair gene testing by letter, rather than a face-to-face genetic counseling feedback session, has been investigated. Other modalities include the development of patient assessments or checklists, CD-ROM programs, and interactive computer programs.
A prospective study evaluated the effects of a CD-ROM decisional support aid for microsatellite instability (MSI) tumor testing in 239 colorectal cancer patients who met the revised Bethesda criteria but who did not meet the Amsterdam criteria. The study also tested a theoretical model of factors influencing decisional conflict surrounding decisions to pursue MSI tumor testing. Within the study, half of the sample was randomly assigned to receive a brief description of MSI testing within the clinical encounter, and the other half was provided the CD-ROM decisional support aid in addition to the brief description. The CD-ROM and brief description intervention increased knowledge about MSI testing more than the brief description alone did. As a result, participants felt more prepared to make a decision about the test and had increased perceived benefits of MSI testing.
Other innovative strategies include educational materials and interactive computer technology. In one study, a 13-page color communication aid using a diverse format for conveying risk, including graphic representations and verbal descriptions, was developed. The authors evaluated the influence of the communication aid in 27 women who were at high risk of having a BRCA1/BRCA2 pathogenic variant. They compared these women with a sample of 107 women who received standard genetic counseling. Improvements in genetic knowledge and accuracy of risk perception were documented in those who had read the aid. There were no differences in anxiety or depression between groups. Personalized, interactive electronic materials have also been developed to aid in genetic education and counseling. In one study, an interactive computer education program available prior to the genetic counseling session was compared with genetic counseling alone in women undergoing counseling for BRCA1/BRCA2 testing. Use of the computer program prior to genetic counseling reduced face-time with the genetic counselor, particularly for those at lower risk of a BRCA1/BRCA2 pathogenic variant. Many of the counselors reported that their client’s use of the computer program allowed them to be more efficient and to reallocate time spent in the sessions to clients’ unique concerns.
Videoconferencing is an innovative strategy to facilitate genetic counseling sessions with clients who cannot travel to specialized clinic settings. In 37 individuals in the United Kingdom, real-time video conferencing was compared with face-to-face counseling sessions; both methods were found to improve knowledge and reduce anxiety levels. Similarly, teleconferencing sessions, in which the client and genetic specialists talked with each other in real time, were used in rural Maine communities for pediatric genetic consults. These sessions were used to convey genetic information and developmental delays. These sessions resulted in comparable decision-making confidence and session satisfaction when contrasted with in-person consultations. An Australian study compared the experiences of 106 women who received hereditary breast and ovarian cancer (HBOC) genetic counseling via videoconferencing with the experiences of 89 women who received counseling face to face. Pre- and 1-month postcounseling assessments revealed no significant differences in knowledge gains, satisfaction, cancer-specific anxiety, generalized anxiety, depression, and perceived empathy of the genetic counselor.
Posttest Education and Result Notification
Posttest counseling may include consideration of the implications of the test results for other family members. It has been suggested that some individuals affected by an inherited disorder agree to have genetic testing performed in order to acquire information that could be shared with family members. There is evidence that implementation of a follow-up counseling program with the proband, after test results are revealed, will significantly increase the proportion of relatives informed of their genetic risk. Follow-up counseling may include telephone conversations with the proband verifying which family members have been contacted and an offer to assist with conveying information to family members. Some experts have suggested that if a test result is positive, plans should be made at this time for the notification, education, and counseling of other relatives based on the test result of the individual. Written materials, brochures, or personal letters may aid people in informing the appropriate relatives about genetic risk.
When a test result is negative, the posttest session may be briefer. It is important, however, to discuss genetic, medical, and psychological implications of a negative result in a family with a known pathogenic variant. For example, it is essential that the person understand that the general population risks for relevant cancer types still apply; additionally, the person’s individual risk of cancer may still be influenced by other risk factors and family history from the other side of the person's family. Furthermore, people may feel distress even when a test is negative. This outcome has been documented in the context of BRCA1/BRCA2 pathogenic variant testing and may also be anticipated in other cancer susceptibility testing. Posttest results discussion of such distress may lead to referral for additional counseling in some cases.
Many individuals benefit from follow-up counseling and consultation with medical specialists after disclosure of genetic test results. This provides an opportunity for further discussion of feelings about their risk statuses, options for risk management (including screening and detection procedures), and implications of the test results for other family members. For example, a standard-of-care randomized controlled trial of 100 female BRCA1/BRCA2 carriers found positive psychological outcomes and improved sleep quality in those who participated in the 12-week Inquiry-Based Stress Reduction (IBSR) intervention. In this study, outcomes were measured 24 to 26 weeks after the IBSR intervention.
Cascade Genetic Testing of Family Members
Cascade genetic testing refers to the process of offering genetic testing to biologic family members who are at risk of inheriting the pathogenic variant previously identified in their relative. The process is repeated as additional pathogenic variant carriers are identified within a family. Cascade testing provides the opportunity to identify carriers of a pathogenic variant prior to cancer presentation, which allows opportunities for cancer prevention, early detection, risk reduction, and ultimately, improved health outcomes. There is wide variation in how cascade testing is conducted internationally; this section currently focuses on cascade testing in the United States.
Uptake of cascade genetic testing
The dissemination of genetic risk information, from the proband to at-risk relatives, is essential for the uptake of cascade testing. Traditionally, this has consisted of discussions regarding the importance of result dissemination to at-risk relatives identified through the proband’s pedigree; this may be supplemented with a letter or educational materials that the proband can use to facilitate disclosure to family members.
Most studies evaluating the uptake of cascade testing have been done in either HBOC or Lynch syndrome. A systematic evidence review including both HBOC and Lynch syndrome studies found that notification of the family pathogenic variant by the proband in HBOC families ranged from 21% to 44%. First-degree relatives (FDRs), females, and close family members were more likely to be informed. Testing rates varied depending on the study, with 15% to 57% undergoing genetic testing. In Lynch syndrome, studies reported higher rates of the proband providing variant notification ranging from 41% to 94%, with one study reporting 70% of FDRs undergoing testing. However, another study of carriers of pathogenic variants in a gynecologic oncology clinic found high rates of disclosure (87% of FDRs) yet a relatively low uptake of testing, particularly for Lynch syndrome compared with HBOC (33% vs. 49%, respectively; P = .02).
Barriers to cascade genetic testing
Communication barriers
Emotional barriers found to influence communication of pathogenic variant results to family members include loss of contact and lack of a close emotional relationship, transmission of guilt, anxiety about cancer risks in relatives, concerns that family members would have difficulty understanding the results, emotional difficulties for the relative receiving the information, and negative impacts on family relationships and dynamics. Similarly, one study found that patients who reported comfort with discussing health information (P = .012) and/or high communication strength within the family (P = .05) were more likely to disclose positive genetic test results for HBOC and Lynch syndrome. In a qualitative study that focused on family communication, ethnically diverse carriers of BRCA1/BRCA2 pathogenic variants received genetic counseling and testing through a county hospital or a tertiary cancer center. This had no influence on overall rates of disclosure of results to family members (73%). However, individuals of African American heritage and individuals of Asian or Pacific Islander heritage were less likely to disclose results (47% and 70%, respectively), when compared with individuals of White heritage (91%). Additionally, African American individuals were less likely to undergo testing (odds ratio, 0.16; 95% confidence interval, 0.06–0.40).
Information interpretation
In a retrospective study of FDRs of a proband with a pathogenic or likely pathogenic variant, 14% of FDRs who were given the information found the information very or somewhat hard to understand. FDR recall of the test result was concordant with the actual test result in 82% of cases; however, 10% were unable to recall the result.
Strategies to facilitate cascade genetic testing
Several strategies aimed at facilitating family dissemination and testing uptake have been studied.
Written material
The Finland Lynch syndrome Registry studied reaching out to at-risk family members through letters. Family members (N = 446) at 50% risk of developing Lynch syndrome from 36 families were notified through letters, which offered genetic counseling and testing for the documented variant in the family. Of the 446 family members, 347 underwent genetic counseling, with 75% of the entire cohort (n = 334) opting for testing.
Group counseling
Establishment of the Family Information Service at Creighton University provided group counseling sessions to at-risk relatives attending an education session. Group sizes ranged from 15 to 75 individuals and sessions were conducted by genetic nurses and counselors in a location near family residences. While an uptake rate of testing was not reported, these sessions considerably reduced the one-on-one health care provider time, thus increasing the capacity of the genetic clinic.
Proband training
Some groups have studied strategies to prepare probands to disseminate genetic test results to at-risk relatives. A randomized controlled study explored training on a six-step communication strategy that consisted of identifying at-risk relatives, selecting the communication method, assessing family member knowledge, sharing the result, responding to reactions, and providing genetic counseling resources. No significant difference in the rate of genetic test result dissemination between intervention (n = 137) and control groups (n = 112) was detected.
A Netherlands group explored the feasibility of a two-phased telephone motivational interviewing intervention conducted by five trained psychosocial workers. Consultands (n = 144) included those with at least one relative eligible for genetic testing or cancer screening. Phase 1 determined the agenda, confirmed which family members needed to be informed with the proband, and explored current and planned result-sharing mechanisms. Phase 2 focused on sharing certain information, building motivation and self-efficacy, and brainstorming solutions to dissemination barriers. Outcomes showed that consultands found this strategy feasible and acceptable. A randomized study is under way to evaluate whether this intervention increases information dissemination.
An Australian randomized controlled trial evaluated the impact of additional telephone genetic counseling support in individuals who had been diagnosed with a genetic condition, had a child diagnosed with a genetic condition, or were found to be carriers of a pathogenic variant. Additional telephone counseling in the intervention group (n = 45) occurred 2 to 3 times over 12 months, which was compared with controls (n = 50). No significant difference in relatives seeking genetic services was detected.
Low- or no-cost genetic testing
One study explored free genetic testing for at-risk relatives. In a BRCA1/BRCA2 pathogenic variant cohort (n = 115) eligible for free at-risk family member testing, 77% disclosed results to all at-risk family members. Only 60% of FDRs and 47% of more-distant relatives underwent genetic testing.
Genetic testing laboratories are also exploring ways to reduce barriers to testing for family members, given that the uptake of cascade testing is low. Some laboratories now offer low-cost self-pay testing or no-cost genetic testing to FDRs within a specified time period (e.g., 90 or more days) of a pathogenic or likely pathogenic variant being identified in a family member. Outcomes from these efforts have not yet been reported.
One study performed cancer predisposition genetic testing on the basis of a diagnosis of a solid tumor cancer in patients unselected for family history or guideline-specified testing. In individuals found to harbor a pathogenic variant, all biological family members of the probands were offered no-cost testing for a 3-month period. Of the eligible families, 17.6% had one or more family members undergo genetic testing. A median of two individuals per family were tested (range, 1–14), although the total number of family members eligible for testing was not reported.
Online direct-to-consumer testing
One direct-to-consumer cancer genetic testing laboratory that performs only a 30-gene panel offered reduced-cost ($50) testing to FDRs of individuals found to harbor a pathogenic variant. Individuals were emailed information about the family testing program, and those interested who applied (n = 1,101) identified the at-risk FDRs and provided their contact information. The laboratory sent emails to FDRs inviting them to undergo testing for the 30-gene panel, which included the pathogenic variants found in their relatives. In 1 year of follow-up, 48% of the invited FDRs underwent testing. Only 12% of FDRs who were tested and found to have a pathogenic variant continued cascade testing of their own FDRs. Notably, 5% of FDRs were found to have pathogenic variants in genes other than the ones found in their family members. Additionally, 16.8% of FDRs were found to have a variant of uncertain significance.
Direct provider-to-family testing
A systematic evidence review studied the dissemination of genetic test results to the probands' family members, using contact information that was provided by probands. Results showed that the number of probands tested was higher than the number of relatives who had been notified of the probands' results. Four studies also found that relatives did not understand the genetic test results; however, when sufficiently informed by a health care provider, most opted to be tested.
Similarly, in a study with 30 probands who had a pathogenic variant, 114 at-risk relatives were identified. Ultimately, probands gave permission for the study coordinators to contact 102 of the at-risk relatives. Of those relatives, 95 of 102 (93%) were successfully contacted by a member of the genetics team, 92 (97%) agreed to genetic counseling, 82 (86%) agreed to testing, and 66 (70%) completed testing for the variant in the family.
When done in a clinical setting, there may be other considerations, such as billing and institutional privacy regulations, that need to be investigated before pursuing direct contact with family members.
Chatbots
Chatbots use artificial intelligence to create an online avatar that can speak back and forth with users, simulating real conversations on the basis of pre-established text-based dialogues. Chatbots are actively being explored as a mechanism to respond to family member cascade testing queries.
In summary, these studies document that relying on probands to notify family members of genetic risk has limitations affecting both information dissemination and the uptake of testing. No one strategy has been shown to be optimal.
Ethical, legal, and social issues
Ethical, legal, and social issues related to cascade testing, such as duty to warn and disclosure to at-risk relatives, are discussed in the Ethical, Legal, and Social Implications section of this summary.
References
- Baker DL, Schuette JL, Uhlmann WR, eds.: A Guide to Genetic Counseling. Wiley-Liss, 1998.
- Hirschhorn K, Fleisher LD, Godmilow L, et al.: Duty to re-contact. Genet Med 1 (4): 171-2, 1999 May-Jun.
- Offit K, Thom P: Ethicolegal aspects of cancer genetics. Cancer Treat Res 155: 1-14, 2010.
- Lipkus IM: Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Making 27 (5): 696-713, 2007 Sep-Oct.
- Ancker JS, Senathirajah Y, Kukafka R, et al.: Design features of graphs in health risk communication: a systematic review. J Am Med Inform Assoc 13 (6): 608-18, 2006 Nov-Dec.
- Schapira MM, Nattinger AB, McHorney CA: Frequency or probability? A qualitative study of risk communication formats used in health care. Med Decis Making 21 (6): 459-67, 2001 Nov-Dec.
- Ghosh K, Crawford BJ, Pruthi S, et al.: Frequency format diagram and probability chart for breast cancer risk communication: a prospective, randomized trial. BMC Womens Health 8: 18, 2008.
- Green MJ, Peterson SK, Baker MW, et al.: Effect of a computer-based decision aid on knowledge, perceptions, and intentions about genetic testing for breast cancer susceptibility: a randomized controlled trial. JAMA 292 (4): 442-52, 2004.
- Fransen M, Meertens R, Schrander-Stumpel C: Communication and risk presentation in genetic counseling. Development of a checklist. Patient Educ Couns 61 (1): 126-33, 2006.
- Wang C, Gonzalez R, Milliron KJ, et al.: Genetic counseling for BRCA1/2: a randomized controlled trial of two strategies to facilitate the education and counseling process. Am J Med Genet A 134 (1): 66-73, 2005.
- Lobb EA, Butow PN, Moore A, et al.: Development of a communication aid to facilitate risk communication in consultations with unaffected women from high risk breast cancer families: a pilot study. J Genet Couns 15 (5): 393-405, 2006.
- Mackay J, Schulz P, Rubinelli S, et al.: Online patient education and risk assessment: project OPERA from Cancerbackup. Putting inherited breast cancer risk information into context using argumentation theory. Patient Educ Couns 67 (3): 261-6, 2007.
- Green MJ, Peterson SK, Baker MW, et al.: Use of an educational computer program before genetic counseling for breast cancer susceptibility: effects on duration and content of counseling sessions. Genet Med 7 (4): 221-9, 2005.
- Coelho JJ, Arnold A, Nayler J, et al.: An assessment of the efficacy of cancer genetic counselling using real-time videoconferencing technology (telemedicine) compared to face-to-face consultations. Eur J Cancer 41 (15): 2257-61, 2005.
- Lea DH, Johnson JL, Ellingwood S, et al.: Telegenetics in Maine: Successful clinical and educational service delivery model developed from a 3-year pilot project. Genet Med 7 (1): 21-7, 2005.
- Voorwinden JS, Jaspers JP, ter Beest JG, et al.: The introduction of a choice to learn pre-symptomatic DNA test results for BRCA or Lynch syndrome either face-to-face or by letter. Clin Genet 81 (5): 421-9, 2012.
- Hall MJ, Manne SL, Winkel G, et al.: Effects of a decision support intervention on decisional conflict associated with microsatellite instability testing. Cancer Epidemiol Biomarkers Prev 20 (2): 249-54, 2011.
- Zilliacus EM, Meiser B, Lobb EA, et al.: Are videoconferenced consultations as effective as face-to-face consultations for hereditary breast and ovarian cancer genetic counseling? Genet Med 13 (11): 933-41, 2011.
- Forrest LE, Burke J, Bacic S, et al.: Increased genetic counseling support improves communication of genetic information in families. Genet Med 10 (3): 167-72, 2008.
- Hamann HA, Smith TW, Smith KR, et al.: Interpersonal responses among sibling dyads tested for BRCA1/BRCA2 gene mutations. Health Psychol 27 (1): 100-9, 2008.
- Landau C, Novak AM, Ganz AB, et al.: Effect of Inquiry-Based Stress Reduction on Well-being and Views on Risk-Reducing Surgery Among Women With BRCA Variants in Israel: A Randomized Clinical Trial. JAMA Netw Open 4 (12): e2139670, 2021.
- Frey MK, Kahn RM, Chapman-Davis E, et al.: Prospective Feasibility Trial of a Novel Strategy of Facilitated Cascade Genetic Testing Using Telephone Counseling. J Clin Oncol 38 (13): 1389-1397, 2020.
- Dheensa S, Fenwick A, Shkedi-Rafid S, et al.: Health-care professionals' responsibility to patients' relatives in genetic medicine: a systematic review and synthesis of empirical research. Genet Med 18 (4): 290-301, 2016.
- Weaver M: The Double Helix: Applying an Ethic of Care to the Duty to Warn Genetic Relatives of Genetic Information. Bioethics 30 (3): 181-7, 2016.
- Menko FH, Ter Stege JA, van der Kolk LE, et al.: The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam Cancer 18 (1): 127-135, 2019.
- Griffin NE, Buchanan TR, Smith SH, et al.: Low rates of cascade genetic testing among families with hereditary gynecologic cancer: An opportunity to improve cancer prevention. Gynecol Oncol 156 (1): 140-146, 2020.
- Landsbergen K, Verhaak C, Kraaimaat F, et al.: Genetic uptake in BRCA-mutation families is related to emotional and behavioral communication characteristics of index patients. Fam Cancer 4 (2): 115-9, 2005.
- Lerman C, Croyle RT, Tercyak KP, et al.: Genetic testing: psychological aspects and implications. J Consult Clin Psychol 70 (3): 784-97, 2002.
- Stoffel EM, Ford B, Mercado RC, et al.: Sharing genetic test results in Lynch syndrome: communication with close and distant relatives. Clin Gastroenterol Hepatol 6 (3): 333-8, 2008.
- Claes E, Evers-Kiebooms G, Boogaerts A, et al.: Communication with close and distant relatives in the context of genetic testing for hereditary breast and ovarian cancer in cancer patients. Am J Med Genet 116A (1): 11-9, 2003.
- Hughes C, Lerman C, Schwartz M, et al.: All in the family: evaluation of the process and content of sisters' communication about BRCA1 and BRCA2 genetic test results. Am J Med Genet 107 (2): 143-50, 2002.
- Kenen R, Arden-Jones A, Eeles R: We are talking, but are they listening? Communication patterns in families with a history of breast/ovarian cancer (HBOC). Psychooncology 13 (5): 335-45, 2004.
- MacDonald DJ: Genetic predisposition testing for cancer: effects on families' lives. Holist Nurs Pract 12 (3): 9-19, 1998.
- McGivern B, Everett J, Yager GG, et al.: Family communication about positive BRCA1 and BRCA2 genetic test results. Genet Med 6 (6): 503-9, 2004 Nov-Dec.
- Sermijn E, Goelen G, Teugels E, et al.: The impact of proband mediated information dissemination in families with a BRCA1/2 gene mutation. J Med Genet 41 (3): e23, 2004.
- Fehniger J, Lin F, Beattie MS, et al.: Family communication of BRCA1/2 results and family uptake of BRCA1/2 testing in a diverse population of BRCA1/2 carriers. J Genet Couns 22 (5): 603-12, 2013.
- Daly MB, Montgomery S, Bingler R, et al.: Communicating genetic test results within the family: Is it lost in translation? A survey of relatives in the randomized six-step study. Fam Cancer 15 (4): 697-706, 2016.
- Aktan-Collan K, Mecklin JP, Järvinen H, et al.: Predictive genetic testing for hereditary non-polyposis colorectal cancer: uptake and long-term satisfaction. Int J Cancer 89 (1): 44-50, 2000.
- Lynch HT: Family information service and hereditary cancer. Cancer 91 (4): 625-8, 2001.
- Montgomery SV, Barsevick AM, Egleston BL, et al.: Preparing individuals to communicate genetic test results to their relatives: report of a randomized control trial. Fam Cancer 12 (3): 537-46, 2013.
- de Geus E, Eijzenga W, Menko FH, et al.: Design and Feasibility of an Intervention to Support Cancer Genetic Counselees in Informing their At-Risk Relatives. J Genet Couns 25 (6): 1179-1187, 2016.
- Hodgson J, Metcalfe S, Gaff C, et al.: Outcomes of a randomised controlled trial of a complex genetic counselling intervention to improve family communication. Eur J Hum Genet 24 (3): 356-60, 2016.
- Finlay E, Stopfer JE, Burlingame E, et al.: Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet Test 12 (1): 81-91, 2008.
- Invitae: Family follow-up testing. 2020. Available online. Last accessed January 17, 2024.
- Color Health, Inc: Color's Family Testing Program. 2021. Available online. Last accessed January 17, 2024.
- Samadder NJ, Riegert-Johnson D, Boardman L, et al.: Comparison of Universal Genetic Testing vs Guideline-Directed Targeted Testing for Patients With Hereditary Cancer Syndrome. JAMA Oncol 7 (2): 230-237, 2021.
- Caswell-Jin JL, Zimmer AD, Stedden W, et al.: Cascade Genetic Testing of Relatives for Hereditary Cancer Risk: Results of an Online Initiative. J Natl Cancer Inst 111 (1): 95-98, 2019.
- Gordon ES, Babu D, Laney DA: The future is now: Technology's impact on the practice of genetic counseling. Am J Med Genet C Semin Med Genet 178 (1): 15-23, 2018.
- Roberts MC, Dotson WD, DeVore CS, et al.: Delivery Of Cascade Screening For Hereditary Conditions: A Scoping Review Of The Literature. Health Aff (Millwood) 37 (5): 801-808, 2018.
Cancer Genetics Service Delivery
Modalities of Genetic Counseling
Cancer risk assessment counseling is a multistep process that traditionally included an in-person pretest and posttest counseling session. In an effort to overcome access barriers, other modalities have been implemented, including group sessions, telephone counseling, and online genetic counseling using remote videoconferencing, which is often referred to as telegenetics. Of these other modalities, only telephone counseling has been examined for noninferiority against in-person genetic counseling in a randomized controlled trial.
Telephone genetic counseling
A systematic review identified 13 published studies that used a randomized controlled trial design to compare pretest and posttest outcomes for in-person genetic counseling with telephone counseling. Knowledge and psychosocial outcomes (e.g., distress) were found to be noninferior, equivalent, or not statistically significant between telephone counseling and in-person counseling. Two studies demonstrated lower testing intention or uptake among participants who received telephone counseling. The majority of studies also found no difference in satisfaction; however, two studies demonstrated higher satisfaction among individuals who received telephone compared with those who received in-person genetic counseling. A subsequent study examined several dimensions of patient perceptions of genetic counseling among participants of a randomized trial of telephone versus in-person genetic counseling. In the 2-week period after their pretest genetic counseling appointment, participants who had telephone-based counseling were more likely to rate it as convenient; however, they also reported lower levels of support and emotional recognition by the counselor. There were no differences in overall satisfaction. Exploratory analysis demonstrated that racial and ethnic minority participants reported lower perceptions of counselor support with in-person counseling when compared with telephone counseling. The opposite was observed for non-Hispanic White participants. Additional studies are needed to confirm these findings given the small sample size. (The studies were conducted prior to the adoption of multigene panel testing.)
Another group reported results of a study in which all participants (N = 1,178) received in-person pretest counseling at one of five participating sites. Those participants willing to be randomized had their results disclosed by telephone (n = 401) or in person (n = 418). Notably, 30% of participants in this study had multigene panel testing. In this trial, telephone disclosure was noninferior to in-person results disclosure when comparing primary psychosocial outcomes (e.g., general and state anxiety). In primary analysis, knowledge did not meet the threshold of noninferiority without imputing missing data. Secondary outcomes related to cancer distress, depression, uncertainty, satisfaction with genetic testing, and behavioral intentions for risk management strategies were not statistically significant between groups.
Video-assisted genetic counseling
Studies have also examined the use of online genetic counseling using remote videoconferencing (telegenetics) as an alternative to in-person genetic counseling and demonstrated increases in patient knowledge, high levels of satisfaction, and minimal negative psychosocial outcomes.
Genetic Service Delivery Models
Emerging approaches to delivering clinical genetic services have been examined to facilitate greater access to genetic counseling and testing. These approaches have been utilized to streamline the process by which high-risk or affected individuals are identified and referred to genetic services. These service delivery models vary in the processes by which patients receive genetic education, counseling, and testing, with genetic counseling increasingly taking place only after genetic testing has already occurred.
Several factors have contributed to the provision of genetic testing without pretest genetic counseling. These factors include: (1) expansion of genetic testing criteria, resulting in increased demand for genetic testing; (2) more indications for testing at the time of cancer diagnosis, given that the identification of a pathogenic variant may affect treatment options (e.g., poly [ADP-ribose] polymerase [PARP] inhibitors in BRCA1/BRCA2 positive patients with metastatic human epidermal growth factor receptor 2–negative breast cancer, ovarian cancer, or pancreatic cancer); (3) increasing numbers of patients who undergo tumor genomic testing to guide treatment, which may be followed by confirmatory germline testing; (4) increasing availability of universal testing (e.g., for ovarian, pancreatic, and prostate cancer patients) and consumer-directed genetic testing. Some indications have resulted in patients being offered genetic testing by their health care providers in a nongenetics environment (e.g., by primary care providers [PCP], surgeons, or oncologists), which may then be followed by posttest result interpretation and counseling by the provider or via a genetics specialist.
Targeted outreach and testing of high-risk populations
High-risk populations, such as those of Ashkenazi Jewish descent, may be offered genetic testing through targeted outreach and population screening programs without pretest counseling or a streamlined education process that provides written or other materials. In an Israeli study that offered population screening via proactive outreach and self-referral, BRCA1/BRCA2 testing uptake was 67%, and satisfaction with the population screening approach was greater than 90% at both 1 week and 6 months posttesting. Posttest genetic counseling was offered in person to BRCA1/BRCA2 carriers with significant family histories of cancer. Letters detailing test results and general screening recommendations were sent to noncarriers with limited family histories. Posttest counseling compliance was 100% for carriers and 87% for noncarriers with suggestive family histories. Notably, gender differences in compliance were found among noncarriers (89% for women vs. 78% for men; P = .01).
A U.S. study deployed community outreach and online interactive pretest education (via a chatbot and video education) for individuals with Ashkenazi Jewish ancestry (i.e., had at least one grandparent who was Ashkenazi Jewish). Participants were then offered genetic testing for the three Ashkenazi Jewish founder pathogenic variants in BRCA1/BRCA2. The study reported a genetic testing uptake rate of 79% among participants. However, only 8% of those who visited the study website enrolled. This suggests that those who enrolled after pretest education were motivated and likely to proceed with genetic testing. Like in the Israeli study, genetic test results were delivered to participants by a clinician (cancer specialist or the participant's PCP) when results were positive or negative and when the participants had an increased risk of carrying a BRCA1/BRCA2 pathogenic variant (based on a familial BRCA1/BRCA2 pathogenic variant or significant family histories of cancer). Negative test results were mailed to all other participants. Of note, participants who tested negative and had significant family histories displayed low adherence to study recommendations for additional genetic testing.
Universal germline genetic testing approaches in oncology
Universal germline genetic testing is now standard practice for patients with certain cancers, such as epithelial ovarian, exocrine pancreatic, and metastatic prostate cancers. For more information on hereditary prostate cancer genetic testing, see the Indications for Prostate Cancer Germline Genetic Testing section in Genetics of Prostate Cancer. For more information on hereditary ovarian cancer genetic testing, see the Indications for hereditary breast and gynecologic cancers genetic testing section in Genetics of Breast and Gynecologic Cancers.
Long-standing guidelines for universal germline genetic testing exist for patients with ovarian cancer. National guidelines in the United States and international guidelines recommend offering genetic testing to all women with ovarian cancer. There are two primary reasons for the endorsement of universal testing in this patient population, given that up to 15% of ovarian cancer patients harbor a pathogenic variant in BRCA1 or BRCA2: (1) to identify patients who may benefit from targeted therapy with a PARP inhibitor, such as olaparib; and (2) to facilitate the identification of at-risk relatives through the process of cascade testing.
In response, various efforts have been made to implement universal germline testing for cancer patients, including embedding genetic services into patients' oncology and oncology-mediated care.
Embedded genetic services
Studies have examined the impact of embedding a cancer genetic counselor on site at gynecologic oncology clinics to increase referral to genetics and increase the use of genetic counseling among affected women. Referral rates and genetic counseling uptake improved (up to 85%) following the incorporation of an on-site genetic counselor. Studies reported that patients had shorter appointment visit lengths with the genetic counselor. Patients also had shorter intervals between being referred to the clinic and completing their genetic consultations.
Oncology-mediated or mainstreamed models of care
Other universal testing efforts have focused on oncology-mediated or mainstreamed models of care wherein oncology physicians provide pretest education and counseling, obtain informed consent, conduct genetic testing, and return negative genetic test results, with possible triaging the return of positive or variants of unknown significance results to genetic counselors. One study reported that patients were highly satisfied (>99%) with oncologist-led genetic counseling and testing.
A systematic review described the results from 11 studies of oncology provider–led germline cancer genetic testing. Most studies (10/11) involved BRCA1/BRCA2 testing in the context of breast or ovarian cancer. Overall, oncologist-led genetic testing resulted in a shorter turnaround time for test results, a higher genetic testing uptake, and similar psychological outcomes when compared with genetic testing conducted via genetic counseling referral. Patients also reported valuing the convenience and continuity of care with a familiar, trusted provider. Patient satisfaction with oncologist-led counseling and testing was high. Oncologists have also reported favorable experiences regarding this counseling approach and find it acceptable. However, some studies reported that patients who received pretest genetic counseling by a nongenetics provider were likely to remember fewer elements that were discussed during the informed consent process. Furthermore, some patients did not recall having a pretest informed consent discussion. Other studies have similarly reported that patients learned less and gave less favorable evaluations regarding the helpfulness of the information that they received from oncology-mediated care.
Mainstreamed models in primary care
Mainstreaming approaches are also being deployed in non-oncology practices. For example, in a study of five community obstetrics and gynecologic practices, clinicians were trained in hereditary cancer risk assessment. Clinics also modified patient screening practices and workflows. After 8 weeks of deploying the modified workflow, 92.8% (3,811/4,107) of patients were assessed for hereditary cancer risk, and 23.8% (906/3,811) of those assessed met National Comprehensive Cancer Network (NCCN) guidelines for genetic testing. Among those who met NCCN guidelines, 89.7% (813/906) were offered genetic testing by their clinicians. Overall, 26.7% (219/813) of the women provided samples for testing and 20% (165/813) received their results. Satisfaction with the process was high (~97%). Notably, patients who were offered genetic testing during their clinic visits (i.e., point-of-care testing) were more likely to submit a sample (98.1%) than those who were referred to a nurse practitioner to have genetic testing within 2 weeks (40.7%). Results in this study were delivered by the treating clinician.
Triaged services using nongenetics providers
Nongenetics providers who receive training in cancer genetics are increasingly using triaged models to increase access to cancer genetics services. These providers may be engaged at different points along the risk assessment, counseling, and testing time line. In one example, nurses were trained to provide basic risk assessment and offer BRCA testing to patients in an effort to increase access to genetic service providers in rural settings. Family histories collected via a paper screener administered in mammography or oncology sites were reviewed and triaged by the genetic counselor based on risk status, and subsequent counseling (regarding risk) was provided by either the nurse or the genetic counselor. A fourfold increase in the number of patients seen at the site was observed over a 2-year period. In another study, patient intake workflow was modified to include genetic counseling assistants. In this study, genetic counseling assistants provided pretest education, performed informed consent for testing, and ordered genetic testing for new patients with pancreatic cancer, whereas genetic counselors only reviewed and returned test results. The genetic testing rate increased from 19% to 71% after the clinic began using genetic counseling assistants in their workflow.
References
- Ormond K: Recommendations for telephone counseling. J Genet Couns 9 (1): 63-71, 2000.
- Sangha K: Assessment of the effectiveness of genetic counseling by telephone compared to a clinic visit. J Genet Couns 12 (2): 171-84, 2003.
- Calzone KA, Prindiville SA, Jourkiv O, et al.: Randomized comparison of group versus individual genetic education and counseling for familial breast and/or ovarian cancer. J Clin Oncol 23 (15): 3455-64, 2005.
- Jenkins J, Calzone KA, Dimond E, et al.: Randomized comparison of phone versus in-person BRCA1/2 predisposition genetic test result disclosure counseling. Genet Med 9 (8): 487-95, 2007.
- Peshkin BN, Demarco TA, Graves KD, et al.: Telephone genetic counseling for high-risk women undergoing BRCA1 and BRCA2 testing: rationale and development of a randomized controlled trial. Genet Test 12 (1): 37-52, 2008.
- Zilliacus EM, Meiser B, Lobb EA, et al.: Women's experience of telehealth cancer genetic counseling. J Genet Couns 19 (5): 463-72, 2010.
- Rothwell E, Kohlmann W, Jasperson K, et al.: Patient outcomes associated with group and individual genetic counseling formats. Fam Cancer 11 (1): 97-106, 2012.
- Platten U, Rantala J, Lindblom A, et al.: The use of telephone in genetic counseling versus in-person counseling: a randomized study on counselees' outcome. Fam Cancer 11 (3): 371-9, 2012.
- Benusiglio PR, Di Maria M, Dorling L, et al.: Hereditary breast and ovarian cancer: successful systematic implementation of a group approach to genetic counselling. Fam Cancer 16 (1): 51-56, 2017.
- Fournier DM, Bazzell AF, Dains JE: Comparing Outcomes of Genetic Counseling Options in Breast and Ovarian Cancer: An Integrative Review . Oncol Nurs Forum 45 (1): 96-105, 2018.
- Schwartz MD, Valdimarsdottir HB, Peshkin BN, et al.: Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol 32 (7): 618-26, 2014.
- Kinney AY, Steffen LE, Brumbach BH, et al.: Randomized Noninferiority Trial of Telephone Delivery of BRCA1/2 Genetic Counseling Compared With In-Person Counseling: 1-Year Follow-Up. J Clin Oncol 34 (24): 2914-24, 2016.
- Kinney AY, Butler KM, Schwartz MD, et al.: Expanding access to BRCA1/2 genetic counseling with telephone delivery: a cluster randomized trial. J Natl Cancer Inst 106 (12): , 2014.
- Athens BA, Caldwell SL, Umstead KL, et al.: A Systematic Review of Randomized Controlled Trials to Assess Outcomes of Genetic Counseling. J Genet Couns 26 (5): 902-933, 2017.
- Peshkin BN, Kelly S, Nusbaum RH, et al.: Patient Perceptions of Telephone vs. In-Person BRCA1/BRCA2 Genetic Counseling. J Genet Couns 25 (3): 472-82, 2016.
- Bradbury AR, Patrick-Miller LJ, Egleston BL, et al.: Randomized Noninferiority Trial of Telephone vs In-Person Disclosure of Germline Cancer Genetic Test Results. J Natl Cancer Inst 110 (9): 985-993, 2018.
- Otten E, Birnie E, Ranchor AV, et al.: Telegenetics use in presymptomatic genetic counselling: patient evaluations on satisfaction and quality of care. Eur J Hum Genet 24 (4): 513-20, 2016.
- Buchanan AH, Datta SK, Skinner CS, et al.: Randomized Trial of Telegenetics vs. In-Person Cancer Genetic Counseling: Cost, Patient Satisfaction and Attendance. J Genet Couns 24 (6): 961-70, 2015.
- Bradbury A, Patrick-Miller L, Harris D, et al.: Utilizing Remote Real-Time Videoconferencing to Expand Access to Cancer Genetic Services in Community Practices: A Multicenter Feasibility Study. J Med Internet Res 18 (2): e23, 2016.
- Solomons NM, Lamb AE, Lucas FL, et al.: Examination of the Patient-Focused Impact of Cancer Telegenetics Among a Rural Population: Comparison with Traditional In-Person Services. Telemed J E Health 24 (2): 130-138, 2018.
- Lieberman S, Tomer A, Ben-Chetrit A, et al.: Population screening for BRCA1/BRCA2 founder mutations in Ashkenazi Jews: proactive recruitment compared with self-referral. Genet Med 19 (7): 754-762, 2017.
- Metcalfe KA, Poll A, Royer R, et al.: Screening for founder mutations in BRCA1 and BRCA2 in unselected Jewish women. J Clin Oncol 28 (3): 387-91, 2010.
- Metcalfe KA, Mian N, Enmore M, et al.: Long-term follow-up of Jewish women with a BRCA1 and BRCA2 mutation who underwent population genetic screening. Breast Cancer Res Treat 133 (2): 735-40, 2012.
- Gronwald J, Huzarski T, Byrski T, et al.: Direct-to-patient BRCA1 testing: the Twoj Styl experience. Breast Cancer Res Treat 100 (3): 239-45, 2006.
- Morgan KM, Hamilton JG, Symecko H, et al.: Targeted BRCA1/2 population screening among Ashkenazi Jewish individuals using a web-enabled medical model: An observational cohort study. Genet Med 24 (3): 564-575, 2022.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 2.2024. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023. Available online with free registration. Last accessed October 31, 2023.
- Society of Gynecologic Oncology: SGO Clinical Practice Statement: Genetic Testing for Ovarian Cancer. 2014. Available online. Last accessed January 17, 2024.
- Marth C, Hubalek M, Petru E, et al.: AGO Austria recommendations for genetic testing of patients with ovarian cancer. Wien Klin Wochenschr 127 (15-16): 652-4, 2015.
- Vergote I, Banerjee S, Gerdes AM, et al.: Current perspectives on recommendations for BRCA genetic testing in ovarian cancer patients. Eur J Cancer 69: 127-134, 2016.
- McCuaig JM, Stockley TL, Shaw P, et al.: Evolution of genetic assessment for BRCA-associated gynaecologic malignancies: a Canadian multisociety roadmap. J Med Genet 55 (9): 571-577, 2018.
- Zhang S, Royer R, Li S, et al.: Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol Oncol 121 (2): 353-7, 2011.
- Kurian AW, Ward KC, Howlader N, et al.: Genetic Testing and Results in a Population-Based Cohort of Breast Cancer Patients and Ovarian Cancer Patients. J Clin Oncol 37 (15): 1305-1315, 2019.
- Lin J, Sharaf RN, Saganty R, et al.: Achieving universal genetic assessment for women with ovarian cancer: Are we there yet? A systematic review and meta-analysis. Gynecol Oncol 162 (2): 506-516, 2021.
- Scheinberg T, Young A, Woo H, et al.: Mainstream consent programs for genetic counseling in cancer patients: A systematic review. Asia Pac J Clin Oncol 17 (3): 163-177, 2021.
- Kentwell M, Dow E, Antill Y, et al.: Mainstreaming cancer genetics: A model integrating germline BRCA testing into routine ovarian cancer clinics. Gynecol Oncol 145 (1): 130-136, 2017.
- Senter L, O'Malley DM, Backes FJ, et al.: Genetic consultation embedded in a gynecologic oncology clinic improves compliance with guideline-based care. Gynecol Oncol 147 (1): 110-114, 2017.
- Bednar EM, Oakley HD, Sun CC, et al.: A universal genetic testing initiative for patients with high-grade, non-mucinous epithelial ovarian cancer and the implications for cancer treatment. Gynecol Oncol 146 (2): 399-404, 2017.
- Rana HQ, Kipnis L, Hehir K, et al.: Embedding a genetic counselor into oncology clinics improves testing rates and timeliness for women with ovarian cancer. Gynecol Oncol 160 (2): 457-463, 2021.
- Colombo N, Huang G, Scambia G, et al.: Evaluation of a Streamlined Oncologist-Led BRCA Mutation Testing and Counseling Model for Patients With Ovarian Cancer. J Clin Oncol 36 (13): 1300-1307, 2018.
- McCuaig JM, Thain E, Malcolmson J, et al.: A Comparison of Patient-Reported Outcomes Following Consent for Genetic Testing Using an Oncologist- or Genetic Counselor-Mediated Model of Care. Curr Oncol 28 (2): 1459-1471, 2021.
- Richardson M, Min HJ, Hong Q, et al.: Oncology Clinic-Based Hereditary Cancer Genetic Testing in a Population-Based Health Care System. Cancers (Basel) 12 (2): , 2020.
- DeFrancesco MS, Waldman RN, Pearlstone MM, et al.: Hereditary Cancer Risk Assessment and Genetic Testing in the Community-Practice Setting. Obstet Gynecol 132 (5): 1121-1129, 2018.
- DeTroye A, Gabbett K, Yi C, et al.: Genetic testing for patients at risk of hereditary breast and ovarian cancer. JAAPA 35 (10): 48-52, 2022.
- Cohen SA, Nixon DM: A collaborative approach to cancer risk assessment services using genetic counselor extenders in a multi-system community hospital. Breast Cancer Res Treat 159 (3): 527-34, 2016.
- Walker EJ, Goldberg D, Gordon KM, et al.: Implementation of an Embedded In-Clinic Genetic Testing Station to Optimize Germline Testing for Patients with Pancreatic Adenocarcinoma. Oncologist 26 (11): e1982-e1991, 2021.
Ethical, Legal, and Social Implications
Duty to Warn: Considerations About Providers’ Disclosure of Patient’s Genetic Information to At-Risk Relatives
The identification of hereditary cancer risks in patients, through a pedigree-based approach, clinical diagnosis, and/or the results of genetic testing, has implications for both patients and their biological family members. One of the major components of genetic counseling, as recommended by many professional medical societies, is to inform patients about familial risk and to encourage discussion with relatives. For more information about disclosing genetic test results to relatives, see the Strategies to facilitate cascade genetic testing section or the Family communication about genetic testing and hereditary risk section in Genetics of Breast and Gynecologic Cancers.
When patients do not inform their at-risk relatives about potentially actionable genetic risks (e.g., pathogenic variants in high-risk genes such as BRCA1 or BRCA2) or do not give their providers permission to share these results, providers may face a dilemma about their duty to warn the relatives. There are several ethical and legal considerations that factor into decisions about what responsibility, if any, providers have to directly inform at-risk relatives about hereditary cancer risks.
If a provider is considering overriding patient confidentiality or consent to directly notify relatives about genetic information, it is important to consider a consultation with one or more of the following: ethicist, ethics committee, legal counsel, privacy officer, and, if applicable, institutional review board to assure adherence to local ethical standards and legal, regulatory, and privacy requirements.
Duty to warn considerations
Patients are encouraged to provide at-risk relatives with genetic testing results that reveal pathogenic or likely pathogenic variants. This is especially encouraged when patients have moderate-to-high risk variants that are associated with increased cancer risk and may guide decisions about cancer screening and risk reduction behaviors.
If a patient with a pathogenic variant declines to notify at-risk relatives, there is debate about whether his/her health care provider has a duty to warn these family members. The concept of duty to warn has sparked much discussion in the medical community. This issue has been examined from legal and ethical perspectives. Medical organizations have also published expert opinions on a provider's duty to warn at-risk relatives. For example, the American Society of Human Genetics (ASHG) outlined circumstances in which it could be permissible for a health care provider to consider directly contacting and notifying at-risk relatives. (The ASHG statement mentioned above was published in 1998, prior to the publication of HIPAA's Privacy Rule. This ASHG statement has not been updated since.) Such circumstances may include the following:
- There is a high likelihood of harm if relatives are not warned.
- The threat of disease risk is foreseeable and imminent.
- The disease or condition is preventable or treatable.
- The patient, despite encouragement, refuses to inform family members.
- The harm of nondisclosure is greater than the harm of disclosure.
- The at-risk relative(s) are identifiable and may include first-, second-, and third-degree relatives.
It is uncommon for providers to breach a patient's confidentiality by informing at-risk relatives about a genetic test result.
It is possible that the patient refuses to inform family members but gives permission for their provider to directly contact their at-risk relatives. In such instances, it is important to document the patient’s consent and consider the optimum methods for communicating with relatives, as well as the provider’s resources to follow-up with such requests. In addition, even when the patient consents to the provider contacting relatives directly, it is important to consider a consultation with one or more of the following: ethicist, ethics committee, legal counsel, privacy officer, and, if applicable, institutional review board to assure adherence to local ethical standards and legal, regulatory, and privacy requirements.
Many providers may not know the identity of at-risk relatives. Providers also may not be able to obtain or confirm a relative's contact information if it was obtained from publicly available resources. Thus, duty to warn dilemmas arise most frequently when a provider or a provider's medical system treats more than one individual from the same family or when a provider has already had contact with a patient's family members.
For more information about informing at-risk relatives, see the Strategies to facilitate cascade genetic testing section or the Family communication about genetic testing and hereditary risk section in Genetics of Breast and Gynecologic Cancers.
Court holdings regarding duty to warn
There are very few legal precedents that guide whether the duty to directly warn family members is the responsibility of the patient or the provider. The two most prominent cases related to hereditary cancer risk, Pate v Threlkel (regarding medullary thyroid cancer) and Safer v Pack (regarding familial adenomatous polyposis), are also dated (1995 and 1996, respectively) and may have the most relevance only in the states in which the cases were adjudicated (Florida and New Jersey, respectively). These cases and the potential implications of their holdings are discussed elsewhere.
Guidelines and legal/legislative frameworks regarding duty to warn
In deciding whether there may be a duty to warn at-risk relatives about hereditary risk, it is important to balance the bioethical constructs of beneficence and nonmaleficence (providing benefit and avoiding harm, respectively) and autonomy with other factors such as professional societies’ recommendations, state and federal legislation, and court holdings from various states. The definition of genetic information (related to hereditary risk) may vary depending on the legal case and the language used in state and federal legislation, although it generally encompasses genetic testing, as well as family history information. The information below pertains to guidance in the United States, as there is variability in international perspectives and policies.
Professional society guidelines regarding duty to warn
Many professional medical societies and government agencies have published their positions and recommendations on communication between a health care provider and a patient's relatives in regard to disclosure of genetic risk. Several organizations such as the American Medical Association, American Society of Clinical Oncology,National Society of Genetic Counselors, and the International Society of Nurses in Genetics recommend that patients who undergo genetic testing disclose the information directly to their at-risk relatives and do not recommend provider notification of relatives without consent. However, the American Society of Human Genetics, which encourages individuals to notify their relatives directly, also provides an explication for criteria where it may be ethically permissible for providers to directly notify at-risk relatives.
Federal and state laws regarding duty to warn
At the federal level, strict nondisclosure policies govern private health information. The Health Insurance Portability and Accountability Act (HIPAA) applies to protected health information in living and deceased individuals. Specifically, the Standards for Privacy of Individually Identifiable Health Information (Privacy Rule, in effect since 2003), states that it is permissible to disclose health information without consent when the public interest is at risk; therefore, under certain conditions, exceptions to the nondisclosure policy include the following:
- There is serious or imminent threat to the health or safety of a person or the public.
- The threat constitutes an imminent, serious threat to an identifiable third party.
- The physician has the capacity to avert significant harm.
In addition, HIPAA contains a minimum necessary standard, which means that entities that are subject to such regulations can only request and receive information relevant to a specific purpose. The type and extent of genetic information that can be released to relatives depends on several factors. These factors include why the genetic data were obtained (i.e., for research purposes or for public health purposes) and if the genetic data have the potential to support medical decision making for cancer treatment. For example, in some instances it may be permissible for the physician of a genetically tested patient to share results with a relative’s physician if they are relevant to the relative's management recommendations. The interpretation of this standard relates to disclosure to another provider, not the at-risk relatives directly.
At the state level, there is significant variability in statutes as they relate to genetic privacy and when, how, by, and to whom genetic information can be released. The National Human Genome Research Institute at the National Institutes of Health maintains the Genome Statute and Legislative Database, which is updated regularly.
If there is a question about whether it is appropriate to breach patient confidentiality to warn relatives, it is important to review these regulations, as well as federal and case law with an ethicist, ethics committee, legal counsel, and/or privacy officer to ensure adherence to local ethical standards and legal, regulatory, and privacy requirements.
Duty to warn considerations in deceased individuals
The section above primarily addresses the duty to warn relatives when a living patient is unwilling to do so. However, concerns also exist about disclosure of genetic testing results from deceased individuals. This concern has arisen in research contexts related to targeted research findings (i.e., findings directly related to the study at hand) or secondary findings, in biobanks, and in clinical contexts. Pragmatic tools for returning research results are available elsewhere.
In clinical practice, the duty to warn about genetic testing results in a deceased individual has arisen after such testing is performed as part of an autopsy (e.g., revealing an inherited cause for sudden cardiac death). However, in the clinical oncology setting, the question about providers disclosing a decedent’s test result to at-risk relatives may occur in several contexts. Examples include the following:
- When cancer patients die before germline genetic testing results became available or before results were disclosed to them, and the result is positive for a pathogenic or likely pathogenic variant in a high-risk gene such as TP53, BRCA1, BRCA2, or those associated with Lynch syndrome.
- When a variant of uncertain significance is upgraded to a likely or known pathogenic variant, and the patient was not alive to receive the updated information.
- When at-risk relatives request the decedent’s testing result to make more informed testing decisions for themselves.
In anticipation of these possible scenarios, some genetics providers may ask patients to sign a form designating which individuals can access their genetic testing results. This form can specify whether this disclosure can occur after death, regardless of whether the patient had received the results. The form contains the relatives’ full names, relationships to the patient, postal addresses, and if possible, mobile phone numbers and email addresses. NCCN recommends discussing the release of genetic test results to relatives during pretest counseling in the event that the patient dies or becomes incapacitated.
HIPAA is a federal law that applies to protected health information in living and deceased individuals. Unless the deceased individual had expressly stated that genetic testing results should not be shared, under HIPAA, after all relevant points of the law have been considered, it may be possible that this information could be shared with relatives.
It is important to contact a privacy officer or a legal counsel before disclosing a deceased individual's test results to relatives. Privacy officers can determine if documentation is required (e.g., demonstrating who the personal representative is/was for the decedent), if specific regulations apply, and if the deceased individual provided permission to release and/or share genetic test results. In addition, an ethicist, an ethics committee, and, if applicable, an institutional review board may be consulted to ensure adherence to local ethical standards and legal, regulatory, and privacy requirements.
Duty to warn versus duty to rescue
Traditionally, duty to warn refers to a provider's potential responsibility to notify a patient’s at-risk biological relatives, such as children and siblings, about a serious hereditary risk. However, more recently, questions have arisen about the duty to warn or duty to rescue the person being tested on the basis of the identification of secondary genomic findings, or genomic testing results that are potentially actionable but were not sought out as part of the indication for testing.
For example, the American College of Medical Genetics and Genomics (ACMG) recommends that pathogenic variants in 73 genes, including 28 genes associated with 16 cancer/neoplastic syndromes should be reported any time an adult or child undergoes clinical genomic sequencing, regardless of the indication. In 2019, the ACMG clarified its position, noting that the list of secondary findings genes was not intended or validated for use in population screening. However, ACMG's working groups are exploring which genetic variants are the most important to disclose to asymptomatic individuals.
The ACMG also recommends that individuals undergo an informed consent process which allows them to opt out of receiving secondary findings. Of note, various clinical programs, research programs, and laboratories have devised their own list of genes in which identified pathogenic variants could be released as secondary findings (for more information, see [eMERGE] and [MyCode/Geisinger]). In many cases, these gene lists are much broader than the one recommended by ACMG. ACMG's secondary findings list does not include several high-to-moderate cancer risk genes for which screening and risk-reduction measures may be recommended.
However, for many of the genes on the ACMG list, the ACMG and others acknowledge the potential uncertainty about penetrance and, therefore, recommended medical management for individuals who test positive without relevant personal or (known) family history. This consideration may add to the complexity of patient-provider decision making about expanding genetic testing to at-risk relatives.
In light of the complexities associated with possible outcomes of genomic sequencing, approaches to consenting patients about the types of results they would like to receive may include a discussion of the range of potential findings as opposed to a description of the medical implications for pathogenic variants in a host of specific genes.
For example, pathogenic variants may be classified as medically actionable, such as those identified in BRCA1/BRCA2 or MSH2. Other variants may be clinically valid but are associated with a range of risk; these variants may have clinical utility limited to specific circumstances, including the following:
- Some genetic variants may be associated with modest increases in cancer risk.
- Pharmacogenomic variants generally do not predict disease risk but have clinical utility for individuals exposed to certain medications.
- Some variants may reveal an individual's carrier status for Mendelian conditions. These results may not be relevant for patients who do not have children or do not plan to have children. However, these results can have reproductive implications for relatives.
- Highly penetrant risk variants may be identified for which few options exist to lessen disease course or risk (e.g., for amyotrophic lateral sclerosis/Lou Gehrig’s disease or early onset Alzheimer disease).
Another consideration is that somatic testing of tumors may reveal pathogenic variants that, if confirmed in the germline, may have implications for both the patient tested (e.g., with respect to systemic treatment of the current cancer and risks for other cancers) and his or her relatives. This concern may also arise in the context of immunohistochemistry (IHC) or microsatellite instability (MSI) testing of colorectal cancer or uterine tumors, in which testing may be performed primarily to guide treatment of the patient, but subsequent germline testing may also determine whether the patient is affected with Lynch syndrome. Considerations about the implications for relatives and the potential benefits of cascade testing in reducing morbidity and mortality from Lynch syndrome are particularly relevant given that universal testing of colorectal and uterine tumors is increasingly performed at the time of diagnosis, which may include tumor sequencing instead of IHC or MSI screening. Thus, tests performed on tumor tissue, particularly when followed by confirmatory germline testing, may raise dual concerns about the duty to rescue (the patient) and a possible duty to warn at-risk relatives. One way to address these concerns is to have patients undergo an informed consent process before any tumor testing to alert them about the importance and implications of germline testing for themselves and their relatives. For more information, see theDuty to warn considerations section.
Employment and Insurance Discrimination
Genetic information obtained from genetic susceptibility tests may have medical, economic, and psychosocial implications for the individual tested and his or her family members. The potential for employment and insurance discrimination is a common concern for individuals considering genetic testing. However, there is limited documentation of employment and insurance discrimination on the basis of hereditary cancer genetic testing results. For more information on discrimination associated with hereditary cancer genetic testing, see the Informed Consent section.
Legal proceedings, federal/state legislation, and recommendations of professional organizations regarding employment and insurance discrimination
State and federal legislation statutes have been developed to prevent the use of genetic information for employment practices, such as hiring, promotion, and salary decisions; and insurance policies, including life and health coverage, by employers, schools, government agencies, and insurers. According to Executive Order 13145, federal departments and agencies are prohibited from discriminating against employees on the basis of genetic test results or information about a request for genetic testing services. Employers and insurers are prohibited from intentionally lowering policy rates by using practices such as screening for individuals who are at risk of becoming ill or dying because of genetic disease susceptibility, such as cancer. These provisions were extended by the Genetic Information Nondiscrimination Act (GINA) in 2008. For more information, see the GINA section. Federal laws, including GINA, do not cover employer-provided life and disability insurance; however, some states do have legislation addressing the use of genetic information for life and disability policies. Current state statutes and bills may be found through NHGRI's Genome Statute and Legislation Database, which is a useful resource for patients to consult before undergoing genetic testing. Examples of relevant legislation regarding genetic information are summarized in Table 2. The information in this table is not comprehensive but provides key points only. See the original sources for more information.
| Law | Coverage Examples | Key Limitations | Protects All Americans? |
|---|---|---|---|
| USPSTF = United States Preventive Services Task Force. | |||
| aAdapted from Leib et al., NHGRI , and FORCE. | |||
| Civil Rights Act of 1964 | Employment only | Does not apply to health insurance | Yes |
| Applies in instances of discrimination on the basis of genetic information if associated with race or ethnic groups | Strong association with a racial or ethnic group for hereditary cancers is rare | ||
| Americans with Disabilities Act of 1990 | Disabilities associated with manifesting genetic information | Does not apply to health insurance | Yes |
| Health Insurance Portability and Accountability Act of 1996 | Group health insurance plans | Does not stop insurers from requiring genetic tests | Yes |
| Genetic information is not defined | |||
| Forbids excluding an individual in a group health plan due to genetic information | Genetic information can be used for plan underwriting | ||
| Forbids premium increases for different group plan members | Disclosure of genetic information is not restricted | ||
| Preexisting conditions cannot include predictive genetic information | Does not apply to individual health plans, unless covered by the portability provision | ||
| Executive Order 13145 of 2000 | Forbids federal employee workplace genetic discrimination | Does not apply to health insurance | No; excludes members of the United States military and anyone who is NOT a federal employee |
| Only applies to federal employees | |||
| Genetic Information Nondiscrimination Act of 2008 (GINA) (Enacted in 2009) | Forbids genetic discrimination in the workplace and in health insurance | Civil suit is restricted to only those who have had all administrative remedies exhausted | No; excludes members of the United States military, veterans obtaining health care through the Veteran’s Administration, and the Indian Health Service |
| Genetic information broadly defined | |||
| Specific to group and individual insurance plans | |||
| Forbids use of genetic information in underwriting | |||
| Forbids requiring genetic testing by employers and insurers | Does not cover life, disability, and long-term care insurance | ||
| Patient Protection and Affordable Care Act (ACA) (Enacted in 2010) | Group or individual health insurance issuers must provide coverage for all individuals who request it | Health plans can set coverage limits on services that are not considered essential | Yes |
| Eliminates preexisting coverage as a reason to exclude coverage | Screening and preventive medicine coverage have some restrictions | ||
| Eliminates annual and lifetime caps on insurance coverage | Genetic counseling and testing coverage does not apply to everyone (e.g., it does not cover men, Lynch syndrome testing, or women who do not meet USPSTF guidelines for BRCA1/BRCA2 testing) | Yes | |
| Caps out-of-pocket costs for health care | |||
| Covers, without a copayment, some cancer screening and preventive services | |||
| Covers genetic counseling and BRCA1/BRCA2 testing for women who meet certain criteria | |||
Genetic Information Nondiscrimination Act of 2008 (GINA)
This U.S. federal law contains many protections against discrimination based on genetic information. Examples of specific provisions are as follows:
- Prohibits access to individuals’ personal genetic information by insurance companies and by employers.
- Prohibits insurance companies from requesting that applicants for group or individual health coverage plans be subjected to genetic testing or screening and prohibits them from discriminating against health plan applicants on the basis of individual genetic information.
- Prohibits employers from using genetic information to refuse employment and prohibits them from collecting employees’ personal genetic information without their explicit consent.
- Prohibits employment agencies from failing or refusing to refer a candidate on the basis of genetic information.
- Prohibits labor organizations from refusing membership on the basis of a member's genetic make-up.
- Does not mandate coverage for medical tests or treatments.
- Does not prohibit medical underwriting based on current health status.
- Does not limit a treating health provider, including those employed by or affiliated with health plans, from requesting or notifying individuals about genetic tests.
- Does not prohibit occupational testing for toxic monitoring programs, employer-sponsored wellness programs, administration of federal and state family and medical leave laws, and certain cases of inadvertent acquisition of genetic information.
GINA amends and/or extends coverage of HIPAA, ADA, and the Employee Retirement Income Security Act by including genetic information under medical privacy and confidentiality legislation, and employment and insurance determinations. Additionally, with the passage of GINA, researchers and clinicians can encourage participation in clinical trials and appropriate genetic testing knowing that there are federal protections against discrimination on the basis of the results of genetic testing. GINA established the minimum protection level that must be met in all states. However, for states with more robust legislation in place, GINA does not weaken existing protections provided by state law.
However, GINA has several limitations, including the following:
- GINA does not apply to members of the United States military, to veterans obtaining health care through the Veteran’s Administration, or to the Indian Health Service because the laws amended by GINA do not apply to these groups and programs.
- The legislation does not apply to life insurance, long-term care insurance, or disability insurance. Even though GINA does not provide protection for employer-provided disability and life insurance, some states do encompass these arenas in addition to employment, genetic privacy, health insurance, health insurance enforcement, life, disability, and long-term care. NHGRI's Genome Statute and Legislation Database provides a searchable listing of state statutes and bills related to the following topics: direct-to-consumer genetic testing, employment and insurance nondiscrimination, health insurance coverage, privacy, research, and the use of residual newborn screening specimens.
- GINA’s employment provisions generally do not apply to employers with fewer than 15 employees.
Under GINA, it is permissible for employers to request employees' genetic information for the purposes of voluntary wellness programs. However, employers cannot encourage employees to provide their genetic information; this means that if an employee chooses to give genetic information to the wellness program, they cannot receive an additional reward for doing so. Conversely, if an employee chooses to withhold genetic information, they cannot be penalized. Regulations regarding workplace wellness programs have been amended by the U.S. Equal Employment Opportunity Commission, and they are in the process of further revision. Thus, before providing genetic information to such wellness programs, patients should be informed about current regulations and provisions for privacy and confidentiality.
Exception to protections against employment and insurance discrimination: Military personnel
GINA and other state and federal protections do not extend to genetic testing of active duty military personnel or genetic information obtained from active duty military personnel. In the military, genetic testing provides medical information that is to be used to protect military personnel from potentially harmful duties or exposures that could stimulate or aggravate a health problem. For example, use of certain antimalaria medications in individuals with glucose 6-phosphate dehydrogenase deficiency can result in red blood cell rupture. Therefore, some genetic information may be critical for maintaining the health and safety of military personnel, given the possible stressful occupational environments they may face. In addition, all military personnel provide a DNA sample to be maintained in a repository that can be used for identification purposes.
Results of genetic tests for disease predisposition could influence military eligibility for new enlistments. For current military personnel, genetic test results could influence worldwide eligibility, assignments, and promotions.
Thus, it is important for individuals who are considering enlisting in the military or those who are active duty to determine what specific policies apply to them, and what the implications of genetic testing may be for their current and future military career. In addition, they should be aware of the potential implications of clinical and research genetic testing and possible concerns related to direct-to-consumer genomic testing.
References
- Robson ME, Storm CD, Weitzel J, et al.: American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol 28 (5): 893-901, 2010.
- Robson ME, Bradbury AR, Arun B, et al.: American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. J Clin Oncol 33 (31): 3660-7, 2015.
- National Society of Genetic Counselors: National Society of Genetic Counselors Code of Ethics. Chicago, Il: National Society of Genetic Counselors, 2006. Also available online. Last accessed January 17, 2024.
- International Society of Nurses in Genetics: Brief Statement of Need/Importance: Privacy and Confidentiality of Genetic Information: The Role of the Nurse. Pittsburgh, Pa: International Society of Nurses in Genetics, 2018. Also available online. Last accessed January 17, 2024.
- ASHG statement. Professional disclosure of familial genetic information. The American Society of Human Genetics Social Issues Subcommittee on Familial Disclosure. Am J Hum Genet 62 (2): 474-83, 1998.
- Burke W, Press N: Genetics as a tool to improve cancer outcomes: ethics and policy. Nat Rev Cancer 6 (6): 476-82, 2006.
- Clayton EW, Evans BJ, Hazel JW, et al.: The law of genetic privacy: applications, implications, and limitations. J Law Biosci 6 (1): 1-36, 2019.
- Roberts MC, Dotson WD, DeVore CS, et al.: Delivery Of Cascade Screening For Hereditary Conditions: A Scoping Review Of The Literature. Health Aff (Millwood) 37 (5): 801-808, 2018.
- Florida. Supreme Court: Pate v. Threlkel. Wests South Report 661: 278-82, 1995.
- New Jersey. Superior Court, Appellate Division: Safer v. Estate of Pack. Atl Report 677: 1188-93, 1996.
- Offit K, Groeger E, Turner S, et al.: The "duty to warn" a patient's family members about hereditary disease risks. JAMA 292 (12): 1469-73, 2004.
- Storm C, Agarwal R, Offit K: Ethical and legal implications of cancer genetic testing: do physicians have a duty to warn patients' relatives about possible genetic risks? J Oncol Pract 4 (5): 229-30, 2008.
- Offit K, Thom P: Ethicolegal aspects of cancer genetics. Cancer Treat Res 155: 1-14, 2010.
- Suter S: Legal Challenges in Genetics, Including Duty to Warn and Genetic Discrimination. Cold Spring Harb Perspect Med 10 (4): , 2020.
- Rothstein MA: Reconsidering the duty to warn genetically at-risk relatives. Genet Med 20 (3): 285-290, 2018.
- Dheensa S, Fenwick A, Shkedi-Rafid S, et al.: Health-care professionals' responsibility to patients' relatives in genetic medicine: a systematic review and synthesis of empirical research. Genet Med 18 (4): 290-301, 2016.
- Meggiolaro N, Barlow-Stewart K, Dunlop K, et al.: Disclosure to genetic relatives without consent - Australian genetic professionals' awareness of the health privacy law. BMC Med Ethics 21 (1): 13, 2020.
- Mitchell C, Ploem C, Chico V, et al.: Exploring the potential duty of care in clinical genomics under UK law. Med Law Int 17 (3): 158-182, 2017.
- d'Audiffret Van Haecke D, de Montgolfier S: Genetic diseases and information to relatives: practical and ethical issues for professionals after introduction of a legal framework in France. Eur J Hum Genet 26 (6): 786-795, 2018.
- U.S. Department of Health & Human Services: Health Information Privacy: Health Information of Deceased Individuals. Washington, DC: U.S. Department of Health & Human Services, 2013. Available online. Last accessed January 17, 2024.
- Health Insurance Portability and Accountability Act of 1996, Public Law 104-191, 104th Congress. Washington, DC: 1996. Also available online. Last accessed January 17, 2024.
- US Department of Health and Human Services: OCR Privacy Brief: Summary of the HIPAA Privacy Rule. Washington, DC: US Department of Health and Human Services, 2002. Also available online. Last accessed January 17, 2024.
- Evans BJ, Jarvik GP: Impact of HIPAA's minimum necessary standard on genomic data sharing. Genet Med 20 (5): 531-535, 2018.
- Amendola LM, Horike-Pyne M, Trinidad SB, et al.: Patients' Choices for Return of Exome Sequencing Results to Relatives in the Event of Their Death. J Law Med Ethics 43 (3): 476-85, 2015.
- Daniels M, Wathoo C, Brusco L, et al.: Active Disclosure of Secondary Germline Findings to Deceased Research Participants' Personal Representatives: Process and Outcomes. JCO Precis Oncol 1: , 2017.
- Chan B, Facio FM, Eidem H, et al.: Genomic inheritances: disclosing individual research results from whole-exome sequencing to deceased participants' relatives. Am J Bioeth 12 (10): 1-8, 2012.
- Gordon DR, Radecki Breitkopf C, Robinson M, et al.: Should Researchers Offer Results to Family Members of Cancer Biobank Participants? A Mixed-Methods Study of Proband and Family Preferences. AJOB Empir Bioeth 10 (1): 1-22, 2019 Jan-Mar.
- Wolf SM, Scholtes E, Koenig BA, et al.: Pragmatic Tools for Sharing Genomic Research Results with the Relatives of Living and Deceased Research Participants. J Law Med Ethics 46 (1): 87-109, 2018.
- Gatter K: Informed Consent for Genetic Testing in Autopsy. Arch Pathol Lab Med 144 (6): 674-676, 2020.
- Elger BS, Michaud K, Fellmann F, et al.: Sudden death: ethical and legal problems of post-mortem forensic genetic testing for hereditary cardiac diseases. Clin Genet 77 (3): 287-92, 2010.
- Wolf SM, Branum R, Koenig BA, et al.: Returning a Research Participant's Genomic Results to Relatives: Analysis and Recommendations. J Law Med Ethics 43 (3): 440-63, 2015.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 2.2024. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023. Available online with free registration. Last accessed October 31, 2023.
- Meinhardt RA: HHS proposes HIPAA privacy rule changes. Provider 28 (6): 37-8, 41, 2002.
- Koplin JJ, Savulescu J, Vears DF: Why genomics researchers are sometimes morally required to hunt for secondary findings. BMC Med Ethics 21 (1): 11, 2020.
- Miller DT, Lee K, Chung WK, et al.: ACMG SF v3.0 list for reporting of secondary findings in clinical exome and genome sequencing: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med 23 (8): 1381-1390, 2021.
- Miller DT, Lee K, Chung WK, et al.: Correction to: ACMG SF v3.0 list for reporting of secondary findings in clinical exome and genome sequencing: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med 23 (8): 1582-1584, 2021.
- Miller DT, Lee K, Gordon AS, et al.: Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2021 update: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med 23 (8): 1391-1398, 2021.
- Green RC, Berg JS, Grody WW, et al.: ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med 15 (7): 565-74, 2013.
- ACMG Board of Directors: The use of ACMG secondary findings recommendations for general population screening: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med 21 (7): 1467-1468, 2019.
- ACMG Board of Directors: Points to consider for informed consent for genome/exome sequencing. Genet Med 15 (9): 748-9, 2013.
- American College of Medical Genetics and Genomics: Incidental findings in clinical genomics: a clarification. Genet Med 15 (8): 664-6, 2013.
- Wiesner GL, Kulchak Rahm A, Appelbaum P, et al.: Returning Results in the Genomic Era: Initial Experiences of the eMERGE Network. J Pers Med 10 (2): , 2020.
- Schwartz MLB, McCormick CZ, Lazzeri AL, et al.: A Model for Genome-First Care: Returning Secondary Genomic Findings to Participants and Their Healthcare Providers in a Large Research Cohort. Am J Hum Genet 103 (3): 328-337, 2018.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Colorectal. Version 1.2023. Plymouth Meeting, PA: National Comprehensive Cancer Network, 2023. Available with free registration. Last accessed June 28, 2023.
- Bunnik EM, Janssens AC, Schermer MH: A tiered-layered-staged model for informed consent in personal genome testing. Eur J Hum Genet 21 (6): 596-601, 2013.
- Berg JS, Khoury MJ, Evans JP: Deploying whole genome sequencing in clinical practice and public health: meeting the challenge one bin at a time. Genet Med 13 (6): 499-504, 2011.
- Bombard Y, Robson M, Offit K: Revealing the incidentalome when targeting the tumor genome. JAMA 310 (8): 795-6, 2013.
- Dumbrava EI, Brusco L, Daniels M, et al.: Expanded analysis of secondary germline findings from matched tumor/normal sequencing identifies additional clinically significant mutations. JCO Precis Oncol 3: , 2019.
- Seifert BA, O'Daniel JM, Amin K, et al.: Germline Analysis from Tumor-Germline Sequencing Dyads to Identify Clinically Actionable Secondary Findings. Clin Cancer Res 22 (16): 4087-4094, 2016.
- Schrader KA, Cheng DT, Joseph V, et al.: Germline Variants in Targeted Tumor Sequencing Using Matched Normal DNA. JAMA Oncol 2 (1): 104-11, 2016.
- Hampel H: Genetic counseling and cascade genetic testing in Lynch syndrome. Fam Cancer 15 (3): 423-7, 2016.
- Bellcross CA, Bedrosian SR, Daniels E, et al.: Implementing screening for Lynch syndrome among patients with newly diagnosed colorectal cancer: summary of a public health/clinical collaborative meeting. Genet Med 14 (1): 152-62, 2012.
- Hampel H, Pearlman R, Beightol M, et al.: Assessment of Tumor Sequencing as a Replacement for Lynch Syndrome Screening and Current Molecular Tests for Patients With Colorectal Cancer. JAMA Oncol 4 (6): 806-813, 2018.
- Hampel H: Population Screening for Hereditary Colorectal Cancer. Surg Oncol Clin N Am 27 (2): 319-325, 2018.
- Manne SL, Meropol NJ, Weinberg DS, et al.: Facilitating informed decisions regarding microsatellite instability testing among high-risk individuals diagnosed with colorectal cancer. J Clin Oncol 28 (8): 1366-72, 2010.
- Beamer LC, Grant ML, Espenschied CR, et al.: Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol 30 (10): 1058-63, 2012.
- Best M, Butow P, Jacobs C, et al.: Advanced cancer patient preferences for receiving molecular profiling results. Psychooncology 29 (10): 1533-1539, 2020.
- Wauters A, Van Hoyweghen I: Global trends on fears and concerns of genetic discrimination: a systematic literature review. J Hum Genet 61 (4): 275-82, 2016.
- Prince AE, Roche MI: Genetic information, non-discrimination, and privacy protections in genetic counseling practice. J Genet Couns 23 (6): 891-902, 2014.
- Parkman AA, Foland J, Anderson B, et al.: Public awareness of genetic nondiscrimination laws in four states and perceived importance of life insurance protections. J Genet Couns 24 (3): 512-21, 2015.
- Green RC, Lautenbach D, McGuire AL: GINA, genetic discrimination, and genomic medicine. N Engl J Med 372 (5): 397-9, 2015.
- Sankar P: Genetic privacy. Annu Rev Med 54: 393-407, 2003.
- Lowrey KM: Legal and ethical issues in cancer genetics nursing. Semin Oncol Nurs 20 (3): 203-8, 2004.
- Leib JR, Hoodfar E, Haidle JL, et al.: The new genetic privacy law: how GINA will affect patients seeking counseling and testing for inherited cancer risk. Community Oncology 5 (6): 351-4, 2008.
- National Human Genome Research Institute: Genetic Discrimination. Bethesda, Md: National Human Genome Research Institute, 2020. Available online. Last accessed January 17, 2024.
- FORCE: Facing Our Risk of Cancer Empowered: Patient Protection and Affordable Care Act (PPACA). Tampa, Fla: FORCE: Facing Our Risk of Cancer Empowered, 2018. Available online. Last accessed January 17, 2024.
- Asmonga D: Getting to know GINA. An overview of the Genetic Information Nondiscrimination Act. J AHIMA 79 (7): 18, 20, 22, 2008.
- National Human Genome Research Institute: "GINA": The Genetic Information Nondiscrimination Act of 2008: Information for Researchers and Health Care Professionals. Bethesda, MD: National Human Genome Research Institute, 2009. Available online. Last accessed January 17, 2024.
- United States Department of Labor: Frequently Asked Questions Regarding the Genetic Information Nondiscrimination Act. Washington, DC: United States Department of Labor, 2010. Available online. Last accessed January 17, 2024.
- U.S. Equal Employment Opportunity Commission: The Genetic Information Nondiscrimination Act of 2008. Washington, DC: U.S. Equal Employment Opportunity Commission, 2008. Available online. Last accessed January 17, 2024.
- Steck MB: Response to "Workplace Wellness Programs: Educating Patients and Families About Discrimination Via Disclosure of Genetic Information". Clin J Oncol Nurs 23 (2): 124, 2019.
- Hudson KL, Holohan MK, Collins FS: Keeping pace with the times--the Genetic Information Nondiscrimination Act of 2008. N Engl J Med 358 (25): 2661-3, 2008.
- Baruch S, Hudson K: Civilian and military genetics: nondiscrimination policy in a post-GINA world. Am J Hum Genet 83 (4): 435-44, 2008.
- Department of Defense: Office of the Secretary of Defense: Direct-to-Consumer Genetic Testing Advisory for Military Members: DOD memo on DNA testing. Washington, DC: Department of Defense, 2019. Available online. Last accessed January 17, 2024.
Latest Updates to This Summary (03/06/2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Cancer Risk Assessment and Counseling
Revised text to state that discussing how to make the most informed choices about risk, in addition to health care conditions, is a component of the genetic counseling process.
Added Accreditation Council for Genetic Counseling as reference 16 and Hirschberg et al. as reference 17.
Components of the Risk Assessment Process
Revised the list of information that may be collected before and/or during a genetic counseling session to include general psychological history, cognitive deficits in the consultand, patterns of communication within the family, and the health of family members and their relationship statuses.
The Option of Genetic Testing
The subsection Genotyping for pathogenic variants in BRCA1 and BRCA2 was renamed from Genotyping for founder pathogenic variants in BRCA1 and BRCA2.
Added text to state that in 2023, the U.S. Food and Drug Administration authorized 23andMe to market a test that included 44 pathogenic variants in BRCA1 and BRCA2, including the three BRCA1 and BRCA2 founder pathogenic variants that are commonly identified in individuals of Ashkenazi Jewish descent (cited U.S. Food and Drug Administration as reference 57).
This summary is written and maintained by the PDQ Cancer Genetics Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about cancer genetics risk assessment and counseling. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Cancer Genetics Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Cancer Genetics Risk Assessment and Counseling are:
- Kathleen A. Calzone, PhD, RN, AGN-BC, FAAN (National Cancer Institute)
- Suzanne C. O'Neill, PhD (Georgetown University)
- Susan K. Peterson, PhD, MPH (University of Texas, M.D. Anderson Cancer Center)
- John M. Quillin, PhD, MPH, MS (Virginia Commonwealth University)
- Charite Ricker, MS, CGC (University of Southern California)
- Catharine Wang, PhD, MSc (Boston University School of Public Health)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Cancer Genetics Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Cancer Genetics Editorial Board. PDQ Cancer Genetics Risk Assessment and Counseling. Bethesda, MD: National Cancer Institute. Updated
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
The information in these summaries should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.