National Cancer Institute
Post Date: Oct 27, 2022
Merkel cell carcinoma treatment options include surgery, radiation therapy, and chemotherapy. Get detailed information about the diagnosis and treatment of newly diagnosed and recurrent Merkel cell carcinoma in this summary for clinicians.
Merkel Cell Carcinoma Treatment
General Information About Merkel Cell Carcinoma
Merkel cell carcinoma (MCC) was originally described by Toker in 1972 as trabecular carcinoma of the skin. Other names include Toker tumor, primary small cell carcinoma of the skin, primary cutaneous neuroendocrine tumor, and malignant trichodiscoma.
MCC is an aggressive neuroendocrine carcinoma arising in the dermoepidermal junction (see Figure 1), and it is the second most common cause of skin cancer death after melanoma. Although the exact origin and function of the Merkel cell remains under investigation, it is thought to have features of both epithelial and neuroendocrine origin and arise in cells with touch-sensitivity function (mechanoreceptors).
Therapeutic options have been historically limited for patients with advanced disease; however, new immunotherapeutic approaches are associated with durable responses.
Anatomy
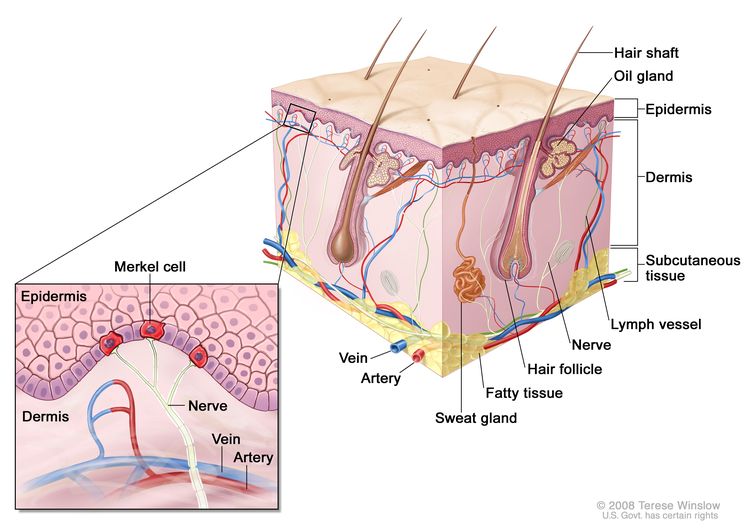 Figure 1. Merkel Cell Anatomy.
Figure 1. Merkel Cell Anatomy.
Epidemiology/Etiology
In Surveillance, Epidemiology, and End Results (SEER) Program data from 1986 to 2001, the age-adjusted U.S. annual incidence of MCC tripled from 0.15 to 0.44 per 100,000, an increase of 8.08% per year. Although this rate of increase is faster than any other skin cancer including melanoma, the absolute number of U.S. cases per year is small. About 1,500 new cases of MCC were expected in the United States in 2007.
Incidence and Mortality
MCC incidence increases progressively with age. There are few cases in patients younger than 50 years, and the median age at diagnosis is about 65 years (see Figure 2). Incidence is considerably greater in White individuals than in Black individuals and slightly greater in men than in women.
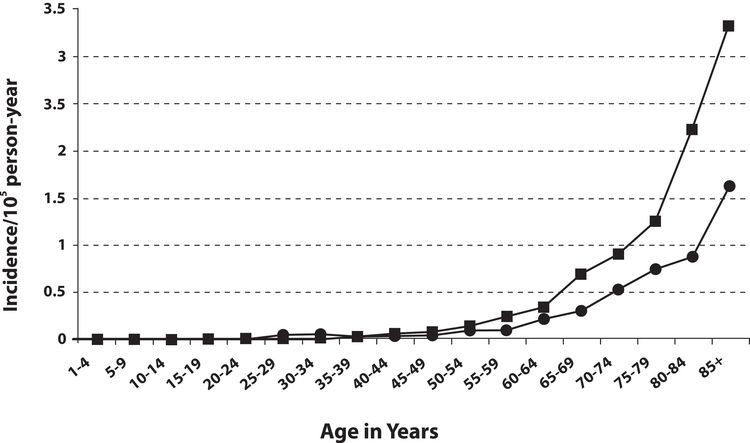 Figure 2. Frequency of MCC by age and sex of men (square) and women (circle). Reprinted from Journal of the American Academy of Dermatology, 49 (5), Agelli M and Clegg L, Epidemiology of primary Merkel cell carcinoma in the United States, pp. 832–41, Copyright (2003), with permission from Elsevier.
Figure 2. Frequency of MCC by age and sex of men (square) and women (circle). Reprinted from Journal of the American Academy of Dermatology, 49 (5), Agelli M and Clegg L, Epidemiology of primary Merkel cell carcinoma in the United States, pp. 832–41, Copyright (2003), with permission from Elsevier.
MCC occurs most frequently in sun-exposed areas of skin, particularly the head and neck, followed by the extremities, and then the trunk. Incidence has been reported to be greater in geographic regions with higher levels of ultraviolet B sunlight.
As of 2013, MCC had an annual incidence of 0.7 per 100,000 in the United States. The incidence has been increasing over the past several decades, almost doubling in the United States between 2000 and 2013. This rise is potentially related to more accurate diagnostic pathology tools, improved clinical awareness of MCC, an aging population, increased sun exposure in susceptible populations, and improved registry tools. The incidence is also higher in immunosuppressed populations (HIV, hematologic malignancies, immunosuppressive medications, etc.). Approximately 25,000 cases of MCC have been recorded in the United States since 2000, including more than 2,200 incident cases reported in 2014 to the National Program of Cancer Registries/SEER combined registries, which captures more than 98% of the U.S. population and the ten most common sites of MCC (see Table 1).
| Anatomic Site | Cases (%) |
|---|---|
| NOS = not otherwise specified; SEER = Surveillance, Epidemiology, and End Results Program. | |
| aAlbores-Saavedra J et al: Merkel cell carcinoma demographics, morphology, and survival based on 3,870 cases: A population-based study. J Cutan Pathol. Reprinted with permission © 2009. Published by Wiley-Blackwell. All rights reserved. | |
| Skin, face | 1,041 (26.9) |
| Skin of upper limb and shoulder | 853 (22.0) |
| Skin of lower limb and hip | 578 (14.9) |
| Skin of trunk | 410 (10.6) |
| Skin of scalp and neck | 348 (9.0) |
| Skin, NOS | 234 (6.0) |
| External ear | 120 (3.1) |
| Eyelid | 98 (2.5) |
| Skin of lip | 91 (2.4) |
| Unknown primary site | 31 (0.8) |
| Total | 3,804 (98.3) |
In various cases series, up to 97% of MCCs arise in the skin. MCC primaries in other sites were very rare, as were MCCs from unknown primary sites.
SEER registry data have shown excess risk of MCC as a first or second cancer in patients with several primary cancers. National cancer registries from three Scandinavian countries have identified a variety of second cancers diagnosed after MCC.
Pathogenesis
Increased incidence of MCC has also been seen in people treated heavily with methoxsalen (psoralen) and ultraviolet A (PUVA) for psoriasis (3 of 1,380 patients, 0.2%). This has also been seen in individuals with chronic immune suppression, especially from chronic lymphocytic leukemia, HIV, and previous solid organ transplant.
In 2008, a novel polyomavirus (Merkel cell polyoma virus [MCPyV]) was first reported in MCC tumor specimens , a finding subsequently confirmed in other laboratories. High levels of viral DNA and clonal integration of the virus in MCC tumors have also been reported along with expression of certain viral antigens in MCC cells and the presence of antiviral antibodies. Not all cases of MCC appear to be associated with MCPyV infection.
MCPyV has been detected at very low levels in normal skin distant from the MCC primary, in a significant percentage of patients with non-MCC cutaneous disorders, in normal-appearing skin in healthy individuals, and in nonmelanoma skin cancers in immune-suppressed individuals. Various methods have been used to identify and quantify the presence of MCPyV in MCC tumor specimens, other non-MCC tumors, blood, urine, and other tissues.
The significance of the new MCPyV findings remains uncertain. The prognostic significance of viral load, antibody titer levels, and the role of underlying immunosuppression in hosts (from disease and medications) are under investigation.
Prevalence of MCPyV appears to differ between MCC patients in the United States and Europe versus Australia. There may be two independent pathways for the development of MCC: one driven by the presence of MCPyV, and the other driven primarily by sun damage, especially as noted in patient series from Australia.
Although no unique marker for MCC has been identified, a variety of molecular and cytogenetic markers of MCC have been reported.
Clinical Presentation
MCC usually presents as a painless, indurated, solitary dermal nodule with a slightly erythematous to deeply violaceous color, and rarely, an ulcer. MCC can infiltrate locally via dermal lymphatics, resulting in multiple satellite lesions. Because of its nonspecific clinical appearance, MCC is rarely suspected before a biopsy is performed. Photographs of MCC skin lesions illustrate its clinical variability.
A mnemonic summarizing typical clinical characteristics of MCC has been proposed:
- A = Asymptomatic.
- E = Expanding rapidly.
- I = Immune suppressed.
- O = Older than 50 years.
- U = UV-exposed skin.
Not all patients have every element in this mnemonic; however, in this study, 89% of patients met three or more criteria, 52% met four or more criteria, and 7% met all five criteria.
Initial Clinical Evaluation
Because local-regional spread is common, newly diagnosed MCC patients require a careful clinical examination that includes looking for satellite lesions and regional nodal involvement.
Tailoring an imaging work-up to the clinical presentation and any relevant signs and symptoms should be considered. There has been no systematic study of the optimal imaging work-up for newly diagnosed patients, and it is not clear if all newly diagnosed patients, especially those with the smallest primaries, benefit from a detailed imaging work-up.
If an imaging work-up is performed, it may include a computed tomography (CT) scan of the chest and abdomen to rule out primary small cell lung cancer as well as distant and regional metastases. Imaging studies designed to evaluate suspicious signs and symptoms may also be recommended. In one series, CT scans had an 80% false-negative rate for regional metastases. Head and neck presentations may require additional imaging. Magnetic resonance imaging has been used to evaluate MCC but has not been studied systematically. Fluorine F 18-fludeoxyglucose positron emission tomography results have been reported in selected cases. Baseline routine blood work has been recommended but has not been studied systematically. There are no known circulating tumor markers specifically for MCC.
Initial Staging Results
The results of initial clinical staging of MCC vary widely in the literature, based on retrospective case series reported over decades. For invasive cancers, 48.6% were localized, 31.1% were regional, and 8.2% were distant.
MCC that presents in regional nodes without an identifiable primary lesion is found in a minority of patients, with the percent of these cases varying among the reported series. Tumors without an identifiable primary lesion have been attributed to either spontaneous regression of the primary or metastatic neuroendocrine carcinoma from a clinically occult site.
Clinical Progression
In a review of patients from 18 case series, 279 of 926 patients (30.1%) developed local recurrence during follow-up, excluding those presenting with distant metastatic disease. These events have been typically attributed to inadequate surgical margins and/or a lack of adjuvant radiation therapy. In addition, 545 of 982 patients (55.5%) had lymph node metastases at diagnosis or during follow-up.
In the same review of 18 case series, the most common sites of distant metastases were distant lymph nodes (60.1%), distant skin (30.3%), lung (23.4%), central nervous system (18.4%), and bone (15.2%). Many other sites of disease have also been reported, and the distribution of metastatic sites varies among case series.
In one series of 237 patients presenting with local or regional disease, the median time-to-recurrence was 9 months (range, 2–70 months). Ninety-one percent of recurrences occurred within 2 years of diagnosis.
Potential Prognostic Factors
The extent of disease at presentation may provide the most useful estimate of prognosis.
Diagnostic procedures, such as sentinel lymph node biopsy, may help distinguish between local and regional disease at presentation. One-third of patients who lack clinically palpable or radiologically visible nodes will have microscopically evident regional disease. Nodal positivity may be substantially lower among patients with small tumors (e.g., ≤1.0 cm).
Many retrospective studies have evaluated the relationship of a wide variety of biological and histological factors to survival and local-regional control.[Level of evidence C2] Many of these reports are confounded by small numbers, potential selection bias, referral bias, short follow-up, no uniform clinical protocol for both staging and treatment, and are underpowered to detect modest differences.
A large, single-institution, retrospective study of 156 MCC patients, with a median follow-up of 51 months (range, 2–224 months), evaluated histological factors potentially associated with prognosis.[Level of evidence C1] Although this report was subject to potential selection and referral bias, both univariate and multivariate analyses demonstrated a relationship between improved cause-specific survival and circumscribed growth pattern versus infiltrative pattern, shallow-tumor depth versus deep-tumor depth, and absence of lymphovascular invasion versus presence of lymphovascular invasion. Adoption of these findings into a global prognostic algorithm awaits independent confirmation by adequately powered studies.
A 2009 study investigated whether the presence of newly identified MCPyV in MCC tumor specimens influenced clinical outcome among 114 Finnish patients with MCC. In this small study, patients whose tumors were MCPyV-positive appeared to have better survival than patients whose tumors were MCPyV-negative.[Level of evidence C2] Standardization of procedures to identify and quantify MCPyV and relevant antibodies is needed to improve understanding of both prognostic and epidemiological questions.
Prognosis
The most significant prognostic parameters for MCC include tumor size and the presence of locoregional or distant metastases. These factors form the basis of the American Joint Committee on Cancer staging system for MCC. Although an increasing primary tumor size correlates with an increased risk of metastatic disease, MCC tumors of any size have significant risk of occult metastasis, supporting the use of sentinel lymph node biopsy for all cases. Additional features of the primary tumor, such as lymphovascular invasion and tumor growth pattern, may also have prognostic significance. Clinically detectable nodal disease is associated with worse outcome than microscopic metastases. Other findings associated with worse prognosis include sheet-like involvement in lymph node metastases and an increasing number of metastatic lymph nodes.
The bulk of MCC literature is from small case series, which are subject to many confounding factors. For this reason, the relapse and survival rates reported by stage vary widely in the literature. In general, lower-stage disease is associated with better overall survival. For more information, see the Potential Prognostic Factors section.
Outcomes from patients presenting with small volume local disease and pathologically confirmed cancer-negative lymph nodes report a cause-specific 5-year survival rate exceeding 90% in one report.[Level of evidence C2]
A tabular summary of treatment results of MCC from 12 series illustrates the difficulty in comparing outcome data among series.
Using the SEER Program registry MCC staging system adopted in 1973, MCC survival data (1973–2006) by stage is summarized below:
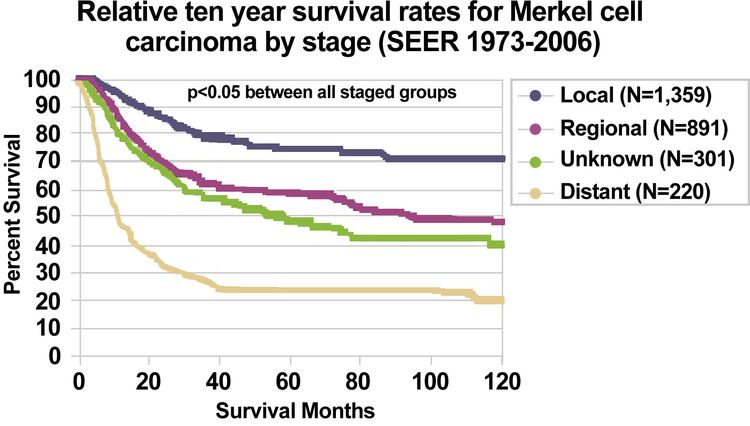 Figure 3. Relative ten-year survival rates for Merkel Cell Carcinoma by stage (SEER 1973–2006). Albores-Saavedra J et al: Merkel cell carcinoma demographics, morphology, and survival based on 3,870 cases: A population-based study. J Cutan Pathol. Reprinted with permission © 2009. Published by Wiley-Blackwell. All rights reserved.
Figure 3. Relative ten-year survival rates for Merkel Cell Carcinoma by stage (SEER 1973–2006). Albores-Saavedra J et al: Merkel cell carcinoma demographics, morphology, and survival based on 3,870 cases: A population-based study. J Cutan Pathol. Reprinted with permission © 2009. Published by Wiley-Blackwell. All rights reserved.
References
- Toker C: Trabecular carcinoma of the skin. Arch Dermatol 105 (1): 107-10, 1972.
- Schwartz RA, Lambert WC: The Merkel cell carcinoma: a 50-year retrospect. J Surg Oncol 89 (1): 5, 2005.
- Agelli M, Clegg LX, Becker JC, et al.: The etiology and epidemiology of merkel cell carcinoma. Curr Probl Cancer 34 (1): 14-37, 2010 Jan-Feb.
- Harms PW: Update on Merkel Cell Carcinoma. Clin Lab Med 37 (3): 485-501, 2017.
- Nghiem P, McKee PH, Haynes HA: Merkel cell (cutaneous neuroendocrine) carcinoma. In: Sober AJ, Haluska FG, eds.: Skin Cancer. BC Decker Inc., 2001, pp 127-141.
- Nghiem P, James N: Merkel cell carcinoma. In: Wolff K, Goldsmith LA, Katz SI, et al., eds.: Fitzpatrick's Dermatology in General Medicine. 7th ed. McGraw-Hill , 2008, pp 1087-94.
- Eng TY, Boersma MG, Fuller CD, et al.: A comprehensive review of the treatment of Merkel cell carcinoma. Am J Clin Oncol 30 (6): 624-36, 2007.
- Medina-Franco H, Urist MM, Fiveash J, et al.: Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol 8 (3): 204-8, 2001.
- Busse PM, Clark JR, Muse VV, et al.: Case records of the Massachusetts General Hospital. Case 19-2008. A 63-year-old HIV-positive man with cutaneous Merkel-cell carcinoma. N Engl J Med 358 (25): 2717-23, 2008.
- Rockville Merkel Cell Carcinoma Group: Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis, and clinical management. J Clin Oncol 27 (24): 4021-6, 2009.
- Calder KB, Smoller BR: New insights into merkel cell carcinoma. Adv Anat Pathol 17 (3): 155-61, 2010.
- Cassler NM, Merrill D, Bichakjian CK, et al.: Merkel Cell Carcinoma Therapeutic Update. Curr Treat Options Oncol 17 (7): 36, 2016.
- Hodgson NC: Merkel cell carcinoma: changing incidence trends. J Surg Oncol 89 (1): 1-4, 2005.
- Agelli M, Clegg LX: Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol 49 (5): 832-41, 2003.
- Young JL, Ward KC, Ries LAG: Cancer of rare sites. In: Ries LAG, Young JL, Keel GE, et al., eds.: SEER Survival Monograph: Cancer Survival Among Adults: U. S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, 2007. NIH Pub. No. 07-6215, pp 251-61.
- Miller RW, Rabkin CS: Merkel cell carcinoma and melanoma: etiological similarities and differences. Cancer Epidemiol Biomarkers Prev 8 (2): 153-8, 1999.
- Lemos B, Nghiem P: Merkel cell carcinoma: more deaths but still no pathway to blame. J Invest Dermatol 127 (9): 2100-3, 2007.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al.: Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol 37 (1): 20-7, 2010.
- Heath M, Jaimes N, Lemos B, et al.: Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol 58 (3): 375-81, 2008.
- Paulson KG, Park SY, Vandeven NA, et al.: Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J Am Acad Dermatol 78 (3): 457-463.e2, 2018.
- Ma JE, Brewer JD: Merkel cell carcinoma in immunosuppressed patients. Cancers (Basel) 6 (3): 1328-50, 2014.
- Howard RA, Dores GM, Curtis RE, et al.: Merkel cell carcinoma and multiple primary cancers. Cancer Epidemiol Biomarkers Prev 15 (8): 1545-9, 2006.
- Bzhalava D, Bray F, Storm H, et al.: Risk of second cancers after the diagnosis of Merkel cell carcinoma in Scandinavia. Br J Cancer 104 (1): 178-80, 2011.
- Lunder EJ, Stern RS: Merkel-cell carcinomas in patients treated with methoxsalen and ultraviolet A radiation. N Engl J Med 339 (17): 1247-8, 1998.
- Feng H, Shuda M, Chang Y, et al.: Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319 (5866): 1096-100, 2008.
- Garneski KM, Warcola AH, Feng Q, et al.: Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J Invest Dermatol 129 (1): 246-8, 2009.
- Becker JC, Houben R, Ugurel S, et al.: MC polyomavirus is frequently present in Merkel cell carcinoma of European patients. J Invest Dermatol 129 (1): 248-50, 2009.
- Kassem A, Schöpflin A, Diaz C, et al.: Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res 68 (13): 5009-13, 2008.
- Houben R, Schrama D, Becker JC: Molecular pathogenesis of Merkel cell carcinoma. Exp Dermatol 18 (3): 193-8, 2009.
- Paik JY, Hall G, Clarkson A, et al.: Immunohistochemistry for Merkel cell polyomavirus is highly specific but not sensitive for the diagnosis of Merkel cell carcinoma in the Australian population. Hum Pathol 42 (10): 1385-90, 2011.
- Andres C, Belloni B, Puchta U, et al.: Prevalence of MCPyV in Merkel cell carcinoma and non-MCC tumors. J Cutan Pathol 37 (1): 28-34, 2010.
- Kassem A, Technau K, Kurz AK, et al.: Merkel cell polyomavirus sequences are frequently detected in nonmelanoma skin cancer of immunosuppressed patients. Int J Cancer 125 (2): 356-61, 2009.
- Foulongne V, Dereure O, Kluger N, et al.: Merkel cell polyomavirus DNA detection in lesional and nonlesional skin from patients with Merkel cell carcinoma or other skin diseases. Br J Dermatol 162 (1): 59-63, 2010.
- DeCaprio JA: Does detection of Merkel cell polyomavirus in Merkel cell carcinoma provide prognostic information? J Natl Cancer Inst 101 (13): 905-7, 2009.
- Laude HC, Jonchère B, Maubec E, et al.: Distinct merkel cell polyomavirus molecular features in tumour and non tumour specimens from patients with merkel cell carcinoma. PLoS Pathog 6 (8): , 2010.
- Buck CB, Lowy DR: Immune readouts may have prognostic value for the course of merkel cell carcinoma, a virally associated disease. J Clin Oncol 29 (12): 1506-8, 2011.
- Seattle Cancer Care Alliance: Merkel Cell Carcinoma Information for Patients and Their Physicians: Clinical Photos/Images. Seattle, Wa: Seattle Cancer Care Alliance Skin Oncology Clinic, 2009. Available online. Last accessed September 30, 2022.
- Gupta SG, Wang LC, Peñas PF, et al.: Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: The Dana-Farber experience and meta-analysis of the literature. Arch Dermatol 142 (6): 685-90, 2006.
- Anderson SE, Beer KT, Banic A, et al.: MRI of merkel cell carcinoma: histologic correlation and review of the literature. AJR Am J Roentgenol 185 (6): 1441-8, 2005.
- Iagaru A, Quon A, McDougall IR, et al.: Merkel cell carcinoma: Is there a role for 2-deoxy-2-[f-18]fluoro-D-glucose-positron emission tomography/computed tomography? Mol Imaging Biol 8 (4): 212-7, 2006 Jul-Aug.
- Belhocine T, Pierard GE, Frühling J, et al.: Clinical added-value of 18FDG PET in neuroendocrine-merkel cell carcinoma. Oncol Rep 16 (2): 347-52, 2006.
- Missotten GS, de Wolff-Rouendaal D, de Keizer RJ: Merkel cell carcinoma of the eyelid review of the literature and report of patients with Merkel cell carcinoma showing spontaneous regression. Ophthalmology 115 (1): 195-201, 2008.
- Richetta AG, Mancini M, Torroni A, et al.: Total spontaneous regression of advanced merkel cell carcinoma after biopsy: review and a new case. Dermatol Surg 34 (6): 815-22, 2008.
- Allen PJ, Bowne WB, Jaques DP, et al.: Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol 23 (10): 2300-9, 2005.
- Stokes JB, Graw KS, Dengel LT, et al.: Patients with Merkel cell carcinoma tumors < or = 1.0 cm in diameter are unlikely to harbor regional lymph node metastasis. J Clin Oncol 27 (23): 3772-7, 2009.
- Jabbour J, Cumming R, Scolyer RA, et al.: Merkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage disease--results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann Surg Oncol 14 (6): 1943-52, 2007.
- Henness S, Vereecken P: Management of Merkel tumours: an evidence-based review. Curr Opin Oncol 20 (3): 280-6, 2008.
- Skelton HG, Smith KJ, Hitchcock CL, et al.: Merkel cell carcinoma: analysis of clinical, histologic, and immunohistologic features of 132 cases with relation to survival. J Am Acad Dermatol 37 (5 Pt 1): 734-9, 1997.
- Sandel HD, Day T, Richardson MS, et al.: Merkel cell carcinoma: does tumor size or depth of invasion correlate with recurrence, metastasis, or patient survival? Laryngoscope 116 (5): 791-5, 2006.
- Llombart B, Monteagudo C, López-Guerrero JA, et al.: Clinicopathological and immunohistochemical analysis of 20 cases of Merkel cell carcinoma in search of prognostic markers. Histopathology 46 (6): 622-34, 2005.
- Senchenkov A, Barnes SA, Moran SL: Predictors of survival and recurrence in the surgical treatment of merkel cell carcinoma of the extremities. J Surg Oncol 95 (3): 229-34, 2007.
- Goldberg SR, Neifeld JP, Frable WJ: Prognostic value of tumor thickness in patients with Merkel cell carcinoma. J Surg Oncol 95 (8): 618-22, 2007.
- Heath ML, Nghiem P: Merkel cell carcinoma: if no breslow, then what? J Surg Oncol 95 (8): 614-5, 2007.
- Tai P: Merkel cell cancer: update on biology and treatment. Curr Opin Oncol 20 (2): 196-200, 2008.
- Andea AA, Coit DG, Amin B, et al.: Merkel cell carcinoma: histologic features and prognosis. Cancer 113 (9): 2549-58, 2008.
- Paulson KG, Iyer JG, Tegeder AR, et al.: Transcriptome-wide studies of merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J Clin Oncol 29 (12): 1539-46, 2011.
- Fields RC, Busam KJ, Chou JF, et al.: Recurrence and survival in patients undergoing sentinel lymph node biopsy for merkel cell carcinoma: analysis of 153 patients from a single institution. Ann Surg Oncol 18 (9): 2529-37, 2011.
- Sihto H, Kukko H, Koljonen V, et al.: Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst 101 (13): 938-45, 2009.
- Harms KL, Healy MA, Nghiem P, et al.: Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System. Ann Surg Oncol 23 (11): 3564-3571, 2016.
- Iyer JG, Storer BE, Paulson KG, et al.: Relationships among primary tumor size, number of involved nodes, and survival for 8044 cases of Merkel cell carcinoma. J Am Acad Dermatol 70 (4): 637-643, 2014.
- Schwartz JL, Griffith KA, Lowe L, et al.: Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol 29 (8): 1036-41, 2011.
- Ko JS, Prieto VG, Elson PJ, et al.: Histological pattern of Merkel cell carcinoma sentinel lymph node metastasis improves stratification of Stage III patients. Mod Pathol 29 (2): 122-30, 2016.
- Eng TY, Boersma MG, Fuller CD, et al.: Treatment of merkel cell carcinoma. Am J Clin Oncol 27 (5): 510-5, 2004.
Cellular Classification of Merkel Cell Carcinoma
Although the exact origin and function of the Merkel cell remains under investigation, it is thought to have features of both epithelial and neuroendocrine origin and arise in cells with touch-sensitivity function (mechanoreceptors).
Characteristic histopathological features include dense core cytoplasmic neurosecretory granules on electron microscopy and cytokeratin-20 on immunohistochemistry (see Figure 4).
A panel of immunoreagents (see Figure 4) can distinguish between Merkel cell carcinoma (MCC) and other similar-appearing tumors including neuroendocrine carcinoma of the lung (i.e., small cell carcinoma), lymphoma, peripheral primitive neuroectodermal tumor, metastatic carcinoid tumor, and small cell melanoma.
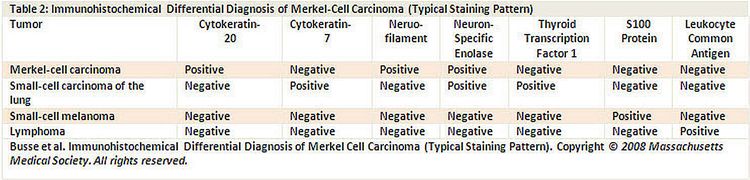 Figure 4. Merkel - Immunohistochemical differential diagnosis of Merkel-Cell Carcinoma (Typical Staining Pattern).
Figure 4. Merkel - Immunohistochemical differential diagnosis of Merkel-Cell Carcinoma (Typical Staining Pattern).
Histologically, MCC has been classified into three distinct subtypes:
- Trabecular: classic pattern, large-cell type, high density or granules on ultrasound examination.
- Intermediate: solid pattern (most common).
- Small cell: diffuse, few high-density granules on ultrasound examination (second most common).
Mixtures of variants are common. Although some small retrospective case series have suggested correlations between certain histological features and outcome, the evidence remains uncertain.
One group has suggested a list of 12 elements that should be described in pathology reports of resected primary lesions and nine elements to be described in pathology reports of sentinel lymph nodes. The prognostic significance of these elements has not been validated prospectively.
If the following data are recorded for every MCC patient, any patient can be staged with the existing or new staging system:
- Size of primary tumor (maximum dimension pathologically or clinically in centimeters).
- Presence/absence of primary tumor invasion into bone, muscle, fascia, or cartilage.
- Presence/absence of nodal metastasis.
- Method used to ascertain status of nodal involvement (clinical or pathological examination).
- Presence/absence of distant metastasis.
The College of American Pathologists has published a protocol for the examination of specimens from patients with MCC of the skin.
For more information, see the Stage Information for Merkel Cell Carcinoma section.
The histological variants of MCC are shown in Figure 5.
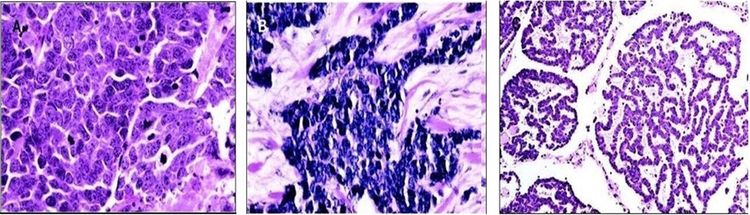 Figure 5. (A) Intermediate variant of MCC showing vesicular, basophilic nuclei with prominent nucleoli and multiple mitoses. (B) Small-cell variant, histologically indistinguishable from bronchial small-cell carcinoma. ©) Trabecular variant is rare and normally only seen as a small component of a mixed variant. Goessling W et al: Merkel Cell Carcinoma, J Clin Oncol, 20 (2), pp. 588–98. Reprinted with permission. © 2009 American Society of Clinical Oncology. All rights reserved.
Figure 5. (A) Intermediate variant of MCC showing vesicular, basophilic nuclei with prominent nucleoli and multiple mitoses. (B) Small-cell variant, histologically indistinguishable from bronchial small-cell carcinoma. ©) Trabecular variant is rare and normally only seen as a small component of a mixed variant. Goessling W et al: Merkel Cell Carcinoma, J Clin Oncol, 20 (2), pp. 588–98. Reprinted with permission. © 2009 American Society of Clinical Oncology. All rights reserved.
References
- Nghiem P, McKee PH, Haynes HA: Merkel cell (cutaneous neuroendocrine) carcinoma. In: Sober AJ, Haluska FG, eds.: Skin Cancer. BC Decker Inc., 2001, pp 127-141.
- Bichakjian CK, Nghiem P, Johnson T, et al.: Merkel Cell Carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 549-62.
- Eng TY, Boersma MG, Fuller CD, et al.: A comprehensive review of the treatment of Merkel cell carcinoma. Am J Clin Oncol 30 (6): 624-36, 2007.
- Medina-Franco H, Urist MM, Fiveash J, et al.: Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol 8 (3): 204-8, 2001.
- Busse PM, Clark JR, Muse VV, et al.: Case records of the Massachusetts General Hospital. Case 19-2008. A 63-year-old HIV-positive man with cutaneous Merkel-cell carcinoma. N Engl J Med 358 (25): 2717-23, 2008.
- Haag ML, Glass LF, Fenske NA: Merkel cell carcinoma. Diagnosis and treatment. Dermatol Surg 21 (8): 669-83, 1995.
- Ratner D, Nelson BR, Brown MD, et al.: Merkel cell carcinoma. J Am Acad Dermatol 29 (2 Pt 1): 143-56, 1993.
- Gould VE, Moll R, Moll I, et al.: Neuroendocrine (Merkel) cells of the skin: hyperplasias, dysplasias, and neoplasms. Lab Invest 52 (4): 334-53, 1985.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al.: Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol 37 (1): 20-7, 2010.
- Alam M: Management of Merkel cell carcinoma: What we know. Arch Dermatol 142 (6): 771-4, 2006.
- Heath ML, Nghiem P: Merkel cell carcinoma: if no breslow, then what? J Surg Oncol 95 (8): 614-5, 2007.
- Andea AA, Coit DG, Amin B, et al.: Merkel cell carcinoma: histologic features and prognosis. Cancer 113 (9): 2549-58, 2008.
- Bichakjian CK, Lowe L, Lao CD, et al.: Merkel cell carcinoma: critical review with guidelines for multidisciplinary management. Cancer 110 (1): 1-12, 2007.
- Rao P, Balzer BL, Lemos BD, et al.: Protocol for the examination of specimens from patients with merkel cell carcinoma of the skin. Arch Pathol Lab Med 134 (3): 341-4, 2010.
- Goessling W, McKee PH, Mayer RJ: Merkel cell carcinoma. J Clin Oncol 20 (2): 588-98, 2002.
Stage Information for Merkel Cell Carcinoma
Previously, five competing staging systems have been used to describe Merkel cell carcinoma (MCC) in most publications.
| First Author | Publication Date | Institution(s) | No. of Patients in Case Series | Dates of Cases |
|---|---|---|---|---|
| MSKCC = Memorial Sloan Kettering Cancer Center; N/A = Not applicable. | ||||
| aThe MSKCC system has evolved over time. MSKCC authors have published one additional case series with 256 patients. | ||||
| Yiengpruksawan | 1991 | MSKCCa | 77 | 1969–1989 |
| Allen | 1999 | MSKCCa | 102 | 1969–1996 |
| Allen | 2005 | MSKCCa | 250 | 1970–2002 |
| American Joint Committee on Cancer | 2017 | N/A | N/A | |
| Clark | 2007 | Westmead Hospital, Sydney, Australia | 110 | |
| Princess Margaret Hospital/University Health Network, Toronto, Canada | ||||
| Sydney Head and Neck Cancer Institute/Royal Prince Alfred Hospital, Sydney, Australia | ||||
These staging systems are highly inconsistent with each other. Stage III disease can mean anything from advanced local disease to nodal disease to distant metastatic disease. Furthermore, all MCC staging systems in use have been based on fewer than 300 patients.
American Joint Committee on Cancer (AJCC) Stage Groupings and TNM Definitions
To address these concerns, a new MCC-specific consensus staging system was developed by the AJCC to define MCC. Before the publication of this new system, the AJCC advocated using the nonmelanoma staging system.
Cancers staged using this staging system include primary cutaneous neuroendocrine carcinoma (MCC).
Clinical Stage Group (cTNM)
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; c = clinical. | ||
| aReprinted with permission from AJCC: Merkel Cell Carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 549–62. | ||
| 0 | Tis, N0, M0 | Tis = In situ primary tumor. |
| N0 = No regional lymph node metastasis detected on clinical and/or radiologic examination. | ||
| M0 = No distant metastasis detected on clinical and/or radiologic examination. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; c = clinical. | ||
| aReprinted with permission from AJCC: Merkel Cell Carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 549–62. | ||
| I | T1, N0, M0 | T1 = Maximum clinical tumor diameter ≤2 cm. |
| N0 = No regional lymph node metastasis detected on clinical and/or radiologic examination. | ||
| M0 = No distant metastasis detected on clinical and/or radiologic examination. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; c = clinical. | ||
| aReprinted with permission from AJCC: Merkel Cell Carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 549–62. | ||
| IIA | T2–3, N0, M0 | T2 = Maximum clinical tumor diameter >2 cm but ≤5 cm. |
| T3 = Maximum clinical tumor diameter >5 cm. | ||
| N0 = No regional lymph node metastasis detected on clinical and/or radiologic examination. | ||
| M0 = No distant metastasis detected on clinical and/or radiologic examination. | ||
| IIB | T4, N0, M0 | T4 = Primary tumor invades fascia, muscle, cartilage, or bone. |
| N0 = No regional lymph node metastasis detected on clinical and/or radiologic examination. | ||
| M0 = No distant metastasis detected on clinical and/or radiologic examination. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; c = clinical. | ||
| aReprinted with permission from AJCC: Merkel Cell Carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 549–62. | ||
| III | T0–4, N1–3, M0 | T0 = No evidence of primary tumor. |
| Tis = In situ primary tumor. | ||
| T1 = Maximum clinical tumor diameter ≤2 cm. | ||
| T2 = Maximum clinical tumor diameter >2 but ≤5 cm. | ||
| T3 = Maximum clinical tumor diameter >5 cm. | ||
| T4 = Primary tumor invades fascia, muscle, cartilage, or bone. | ||
| N1 = Metastasis in regional lymph node(s). | ||
| N2 = In-transit metastasis (discontinuous from primary tumor; located between primary tumor and draining regional nodal basin, or distal to the primary tumor) without lymph node metastasis. | ||
| N3 = In-transit metastasis (discontinuous from primary tumor; located between primary tumor and draining regional nodal basin, or distal to the primary tumor) with lymph node metastasis. | ||
| M0 = No distant metastasis detected on clinical and/or radiologic examination. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; c = clinical. | ||
| aReprinted with permission from AJCC: Merkel Cell Carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 549–62. | ||
| IV | T0–4, Any N, M1 | T0 = No evidence of primary tumor. |
| Tis = In situ primary tumor. | ||
| T1 = Maximum clinical tumor diameter ≤2 cm. | ||
| T2 = Maximum clinical tumor diameter >2 but ≤5 cm. | ||
| T3 = Maximum clinical tumor diameter >5 cm. | ||
| T4 = Primary tumor invades fascia, muscle, cartilage, or bone. | ||
| NX = Regional lymph nodes cannot be clinically assessed (e.g., previously removed for another reason, or because of body habitus). | ||
| N0 = No regional lymph node metastasis detected on clinical and/or radiologic examination. | ||
| N1 = Metastasis in regional lymph node(s). | ||
| N2 = In-transit metastasis (discontinuous from primary tumor; located between primary tumor and draining regional nodal basin, or distal to the primary tumor) without lymph node metastasis. | ||
| N3 = In-transit metastasis (discontinuous from primary tumor; located between primary tumor and draining regional nodal basin, or distal to the primary tumor) with lymph node metastasis. | ||
| M1 = Distant metastasis detected on clinical and/or radiologic examination. | ||
Pathological Stage Group (pTNM)
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; p = pathological. | ||
| aReprinted with permission from AJCC: Merkel Cell Carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 549–62. | ||
| 0 | Tis, pN0, M0 | Tis = In situ primary tumor. |
| pN0 = No regional lymph node metastasis detected on pathological evaluation. | ||
| M0 = No distant metastasis detected on clinical and/or radiologic examination. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; p = pathological. | ||
| aReprinted with permission from AJCC: Merkel Cell Carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 549–62. | ||
| I | T1, pN0, M0 | T1 = Maximum clinical tumor diameter ≤2 cm. |
| pN0 = No regional lymph node metastasis detected on pathological evaluation. | ||
| M0 = No distant metastasis detected on clinical and/or radiologic examination. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; p = pathological. | ||
| aReprinted with permission from AJCC: Merkel Cell Carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 549–62. | ||
| IIA | T2–3, pN0, M0 | T2 = Maximum clinical tumor diameter >2 but ≤5 cm. |
| T3 = Maximum clinical tumor diameter >5 cm. | ||
| pN0 = No regional lymph node metastasis detected on pathological evaluation. | ||
| M0 = No distant metastasis detected on clinical and/or radiologic examination. | ||
| IIB | T4, pN0, M0 | T4 = Primary tumor invades fascia, muscle, cartilage, or bone. |
| pN0 = No regional lymph node metastasis detected on pathological evaluation. | ||
| M0 = No distant metastasis detected on clinical and/or radiologic examination. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; p = pathological. | ||
| aReprinted with permission from AJCC: Merkel Cell Carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 549–62. | ||
| IIIA | T1–4, pN1a(sn) or pN1a, M0 | T1 = Maximum clinical tumor diameter ≤2 cm. |
| T2 = Maximum clinical tumor diameter >2 cm but ≤5 cm. | ||
| T3 = Maximum clinical tumor diameter >5 cm. | ||
| T4 = Primary tumor invades fascia, muscle, cartilage, or bone. | ||
| pN1a(sn) = Clinically occult regional lymph node metastasis identified only by sentinel lymph node biopsy. | ||
| pN1a = Clinically occult regional lymph node metastasis following lymph node dissection. | ||
| M0 = No distant metastasis detected on clinical and/or radiologic examination. | ||
| T0, pN1b, M0 | T0 = No evidence of primary tumor. | |
| pN1b = Clinically and/or radiologically detected regional lymph node metastasis, microscopically confirmed. | ||
| M0 = No distant metastasis detected on clinical and/or radiologic examination. | ||
| IIIB | T1–4, pN1b–3, M0 | T1 = Maximum clinical tumor diameter ≤2 cm. |
| T2 = Maximum clinical tumor diameter >2 cm but ≤5 cm. | ||
| T3 = Maximum clinical tumor diameter >5 cm. | ||
| T4 = Primary tumor invades fascia, muscle, cartilage, or bone. | ||
| pN1b = Clinically and/or radiologically detected regional lymph node metastasis, microscopically confirmed. | ||
| pN2 = In-transit metastasis (discontinuous from primary tumor; located between primary tumor and draining regional nodal basin, or distal to the primary tumor) without lymph node metastasis. | ||
| pN3 = In-transit metastasis (discontinuous from primary tumor; located between primary tumor and draining regional nodal basin, or distal to the primary tumor) with lymph node metastasis. | ||
| M0 = No distant metastasis detected on clinical and/or radiologic examination. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis; p = pathological. | ||
| aReprinted with permission from AJCC: Merkel Cell Carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 549–62. | ||
| IV | T0–4, Any pN, pM1 | T0 = No evidence of primary tumor. |
| T1 = Maximum clinical tumor diameter ≤2 cm. | ||
| T2 = Maximum clinical tumor diameter >2 cm but ≤5 cm. | ||
| T3 = Maximum clinical tumor diameter >5 cm. | ||
| T4 = Primary tumor invades fascia, muscle, cartilage, or bone. | ||
| pNX = Regional lymph nodes cannot be assessed (e.g., previously removed for another reason or not removed for pathological evaluation). | ||
| pN0 = No regional lymph node metastasis detected on pathological evaluation. | ||
| pN1 = Metastasis in regional lymph node(s). | ||
| –pN1a(sn) = Clinically occult regional lymph node metastasis identified only by sentinel lymph node biopsy. | ||
| –pN1a = Clinically occult regional lymph node metastasis following lymph node dissection. | ||
| –pN1b = Clinically and/or radiologically detected regional lymph node metastasis, microscopically confirmed. | ||
| pN2 = In-transit metastasis (discontinuous from primary tumor; located between primary tumor and draining regional nodal basin, or distal to the primary tumor) without lymph node metastasis. | ||
| pN3 = In-transit metastasis (discontinuous from primary tumor; located between primary tumor and draining regional nodal basin, or distal to the primary tumor) with lymph node metastasis. | ||
| pM1 = Distant metastasis microscopically confirmed. | ||
| –pM1a = Metastasis to distant skin, distant subcutaneous tissue, or distant lymph node(s), microscopically confirmed. | ||
| –pM1b = Metastasis to lung, microscopically confirmed. | ||
| pM1c = Metastasis to all other distant sites, microscopically confirmed. | ||
Before the new AJCC consensus staging system was published, the most recent Memorial Sloan Kettering Cancer Center (MSKCC) four-stage system was favored because it was based on the largest number of patients and was the best validated. The stages in the MSKCC system included:
- Stage I: local disease <2 cm.
- Stage II: local disease ≥2 cm.
- Stage III: regional nodal disease.
- Stage IV: distant metastatic disease.
One group has suggested a list of 12 elements that should be described in pathology reports of resected primary lesions and nine elements to be described in pathology reports of sentinel lymph nodes. The prognostic significance of these elements has not been validated prospectively. The 2009 AJCC staging manual also specifies a variety of factors that should be collected prospectively on pathology reports.
References
- Andea AA, Coit DG, Amin B, et al.: Merkel cell carcinoma: histologic features and prognosis. Cancer 113 (9): 2549-58, 2008.
- Yiengpruksawan A, Coit DG, Thaler HT, et al.: Merkel cell carcinoma. Prognosis and management. Arch Surg 126 (12): 1514-9, 1991.
- Allen PJ, Zhang ZF, Coit DG: Surgical management of Merkel cell carcinoma. Ann Surg 229 (1): 97-105, 1999.
- Allen PJ, Bowne WB, Jaques DP, et al.: Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol 23 (10): 2300-9, 2005.
- Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Clark JR, Veness MJ, Gilbert R, et al.: Merkel cell carcinoma of the head and neck: is adjuvant radiotherapy necessary? Head Neck 29 (3): 249-57, 2007.
- Bichakjian CK, Nghiem P, Johnson T, et al.: Merkel Cell Carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 549-62.
- Bichakjian CK, Lowe L, Lao CD, et al.: Merkel cell carcinoma: critical review with guidelines for multidisciplinary management. Cancer 110 (1): 1-12, 2007.
Treatment Option Overview
Merkel cell carcinoma (MCC) is an uncommon tumor. Most clinical management recommendations in the literature are based on case series that describe a relatively small number of patients who were not entered on formal clinical trials, evaluated with uniform clinical staging procedures, treated with uniform treatment protocols, or provided with regular, prescribed follow-up. These reports are also confounded by potential selection bias, referral bias, and short follow-up. They are underpowered to detect modest differences in outcome.
In addition, outcomes of patients with American Joint Committee on Cancer stage I and stage II disease are often reported together. In the absence of results from clinical trials with prescribed work-up, treatments, and follow-up, most MCC patients have been treated using institutional or practitioner preferences that consider the specifics of each case as well as patient preference.
There are two competing philosophies about the most appropriate method of treating MCC. In the first philosophy, MCC is treated like other nonmelanoma skin cancers, with an emphasis on treating local-regional disease with surgery and radiation therapy, as appropriate. In the second philosophy, MCC is treated according to its biological features. This approach makes it analogous to small cell lung cancer, which is assumed to be a systemic disease, and leads to a more routine recommendation of systematic adjuvant chemotherapy.
Surgery for the Primary Lesion
In a review of 18 case series, 279 of 926 patients (30.1%) developed local recurrence during follow-up, excluding those presenting with distant metastatic disease at presentation. These recurrences have been typically attributed to inadequate surgical margins or possibly a lack of adjuvant radiation therapy.
Given the propensity of MCC to recur locally (sometimes with satellite lesions and/or in-transit metastases), wide local excision to reduce the risk of local recurrence has been recommended for patients with clinical stage I or stage II disease.
Recommendations about the optimal minimum width and depth of normal tissue margin to be excised around the primary tumor differ among the various retrospective case series, but this question has not been studied systematically.[Level of evidence C2] No definitive data suggest that extremely wide margins improve overall survival (OS), although some reports suggest that wider margins appear to improve local control.[Level of evidence C2] Frozen-section evaluation of margins may be useful, especially when the tumor is in an anatomical site that is not amenable to wide margins.
Some authors have advocated the use of Mohs micrographic surgery as a tissue-sparing technique. The relapse rate has been reported to be similar to or better than that of wide excision, but comparatively few cases have been treated in this manner and none in randomized controlled trials.[Level of evidence C2]
Regional Lymph Node Surgery
In some case series, local-regional recurrence rates are high when pathological nodal staging is omitted. Surgical nodal staging in clinically negative patients has identified positive nodes in at least 25% to 35% of patients.[Level of evidence C2] In one retrospective series of 213 patients who underwent surgical treatment of the primary tumor and evaluation of the draining nodes, nodal positivity was found in 2 of 54 patients with small tumors (e.g., ≤1.0 cm) and 51 of 159 patients with tumors larger than 1.0 cm.[Level of evidence C2]
The role of elective lymph node dissection (ELND) in the absence of clinically positive lymph nodes has not been studied in formal clinical trials. In small case series, ELND has been recommended for larger primary tumors, tumors with more than ten mitoses per high-power field, lymphatic or vascular invasion, and the small-cell histological subtypes.[Level of evidence C2]
Sentinel lymph node (SLN) biopsy has been suggested as a preferred initial alternative to complete ELND for the proper staging of MCC. SLN biopsy has less morbidity than complete nodal dissection. Furthermore, for MCC sites with indeterminate lymphatic drainage, such as those on the back, SLN biopsy techniques can be used to identify the pertinent lymph node bed(s). If performed, SLN biopsy is done at the time of the wide resection when the local lymphatic channels are still intact.
Several reports have found the use of SLN biopsy techniques in MCC to be reliable and reproducible. However, the significance of SLN positivity remains unclear.
- One meta-analysis of ten case series found that SLN positivity strongly predicted a high short-term risk of recurrence and that subsequent therapeutic lymph node dissection was effective in preventing short-term regional nodal recurrence.
- Another meta-analysis of 12 retrospective case series (only partially overlapping the collection of case series in the previous meta-analysis) found that:[Level of evidence C2]
- SLN biopsy detected MCC spread in one-third of patients whose tumors would have otherwise been clinically and radiologically understaged.
- The recurrence rate was three times higher in patients with a positive SLN biopsy than in those with a negative SLN biopsy (P = .03).
- Between 2006 and 2010, a large, retrospective, single-institutional series of 95 patients (with a total of 97 primary tumors) identified a SLN in 93 instances, and nodal tumor was seen in 42 patients. Immunohistochemical techniques were used to assess node positivity. Various models of tumor and patient characteristics were studied to predict node positivity. There was no subgroup of patients predicted to have lower than 15% to 20% likelihood of SLN positivity, suggesting that SLN biopsy may be considered for all curative patients with clinically negative lymph nodes and no distant metastases.[Level of evidence C2]
- From 1996 to 2010, another retrospective, single-institutional study of 153 patients with localized MCC who underwent SLN biopsy analyzed factors associated with SLN positivity. The best predictors of SLN biopsy positivity were tumor size and lymphovascular invasion.[Level of evidence C2]
In the absence of adequately powered, prospective, randomized clinical trials, the following questions remain:[Level of evidence C2]
- Should every positive SLN biopsy be followed routinely by completion nodal surgery and/or radiation therapy?
- Are outcomes demonstrably improved by routinely adding radiation if lymph node surgery reveals tumor in multiple nodes and/or extracapsular extension and/or lymphovascular invasion?
- Should patients with MCCs smaller than 1 cm routinely undergo sentinel lymph node dissection (SLND)?
- Should patients with negative or omitted nodal work-up routinely undergo local or local-regional radiation therapy?
- Should immunohistochemical staining techniques be used to identify micrometastases in lymph nodes, and is micrometastatic disease in nodes clinically relevant?
Lymph node surgery is primarily used for staging and guiding additional treatment.
Based on a small number of retrospective studies, therapeutic dissection of the regional lymph nodes after a positive SLND appears to minimize, but not totally eliminate, the risk of subsequent regional lymph node recurrence and in-transit metastases.[Level of evidence C2] There are no data from prospective randomized trials demonstrating that definitive regional nodal treatment with surgery improves survival.
Radiation Therapy
Because of the aggressive nature of MCC, its apparent radiosensitivity, and the high incidence of local and regional recurrences (including in-transit metastases after surgery alone to the primary tumor bed), some clinicians have recommended adjuvant radiation therapy to the primary site and nodal basin. Nodal basin radiation in contiguity with radiation to the primary site has been considered, especially for patients with larger tumors, locally unresectable tumors, close or positive excision margins that cannot be improved by additional surgery, and those with positive regional lymph nodes, especially after SLND (stage II).[Level of evidence C2] Several small retrospective series have shown that radiation therapy plus adequate surgery improves local-regional control compared with surgery alone, whereas other series did not show the same results.[Level of evidence C2]
In the absence of adequately powered, prospective, randomized clinical trials, the following questions remain:[Level of evidence C2]
- Should every positive SLN biopsy be followed routinely by completion nodal surgery and/or radiation therapy?
- Are outcomes demonstrably improved by routinely adding radiation only if nodal surgery reveals tumor in multiple lymph nodes and/or extracapsular extension and/or lymphovascular invasion?
- Should all or just certain patients with negative or omitted nodal work-up receive local or local-regional radiation routinely?
Because of the small size of these nonrandomized, retrospective series, the precise benefit from radiation therapy remains unproven.
When recommended, the radiation dose given has been at least 50 Gy to the surgical bed with margins and to the draining regional lymphatics, delivered in 2 Gy fractions. For patients with unresected tumors or tumors with microscopic evidence of spread beyond resected margins, higher doses of 56 Gy to 65 Gy to the primary site have been recommended.[Level of evidence C2] These doses have not been studied prospectively in clinical trials.
Local and/or regional control of MCC with radiation therapy alone has been reported in small, highly selected, nonrandomized case series of patients with diverse clinical characteristics. Typically, these patients have had inoperable primary tumors and/or nodes or were considered medically inappropriate for surgery.[Level of evidence C2]
Retrospective Surveillance, Epidemiology, and End Results (SEER) Program data suggest that adding radiation therapy to surgery adds survival value, but the conclusions are complicated by incomplete patient data, no protocol for evaluation and treatment, and potential sampling bias. Prospective randomized clinical trials are required to assess whether combining surgery with radiation therapy affects survival.[Level of evidence C2]
Immunotherapy
Approximately 70% to 80% of MCC cases in the United States are caused by Merkel cell polyomavirus (MCPyV), and within virus-positive MCCs, the viral oncoproteins (T antigens) are constitutively expressed and growth-promoting. Furthermore, patients with stimulated immune response to MCPyV have better disease outcomes, providing a rationale for the use of immunotherapy.
In a phase II trial, 88 patients with chemotherapy-treated metastatic MCC received avelumab, a human anti–programmed death ligand-1 (PD-L1) monoclonal antibody (10 mg/kg intravenously [IV] every 2 weeks). The objective rate of response was 33%, and 11% of the patients had a complete response. Median response was at 6.1 weeks and was irrespective of MCPyV or PD-L1 status. More importantly, responses were durable, with 74% lasting at least 1 year. As a result, median OS was more than twice the historical median, that is, an OS of 4 to 6 months with second-line chemotherapy. Based on this study, the U.S. Food and Drug Administration approved avelumab in 2017 for the treatment of metastatic MCC, regardless of previous chemotherapy administration.
Pembrolizumab, a humanized IgG4 anti–programmed cell death-1 (PD-1) monoclonal antibody, is being studied in a phase II trial (NCT02267603) of first-line systemic treatment for patients with unresectable stage IIIB or stage IV MCC. The initial report included 26 patients; 16% had a complete response and 40% had a partial response, resulting in an objective response rate of 56%. While the response rate was similar to historical rates with first-line chemotherapy, pembrolizumab responses were more durable, with 86% ongoing at last follow-up.
Nivolumab, another anti-PD-1 antibody, is being studied (NCT02488759) in patients with virus-associated cancers, including MCC. Patients with metastatic MCC were eligible regardless of MCPyV status or previous chemotherapy. Preliminary results reported an ongoing response rate of 87%, with responses in 13 of 15 patients at last follow-up (median follow-up, 6 months). This trial added a second cohort to investigate nivolumab plus ipilimumab (1 mg/kg) in patients with metastatic MCC.
Chemotherapy
A variety of chemotherapy regimens have been used for patients with MCC in the settings of adjuvant, advanced, and recurrent therapy. [Level of evidence C2] Even though no phase III clinical trials have been conducted to demonstrate that adjuvant chemotherapy produces improvements in OS, some clinicians recommend its use in most cases because of the following:
- A biologic analogy is made between MCC and the histologically similar small cell carcinoma of the lung, which is considered a systemic disease.
- The risk of metastases and progression with MCC is high.
- Good initial clinical response rates have been noted with some chemotherapy regimens.
When possible, patients can be encouraged to participate in clinical trials.
From 1997 to 2001, the Trans-Tasman Radiation Oncology Group performed a phase II evaluation of 53 MCC patients with high-risk, local-regional disease. High risk was defined as recurrence after initial therapy, involved lymph nodes, primary tumor larger than 1 cm, gross residual disease after surgery, or occult primary with positive lymph nodes. Therapy included local-regional radiation therapy (50 Gy in 25 fractions), synchronous carboplatin (area under the curve [AUC] 4.5), and IV etoposide (89 mg/m2 on days 1–3 in weeks 1, 4, 7, and 10). Surgery was not standardized for either the primary tumor or the lymph nodes, and 12 patients had close margins, positive margins, or gross residual disease. Twenty-eight patients had undissected nodal beds, and the remainder had a variety of nodal surgeries. With a median follow-up of 48 months, the 3-year OS rate was 76%, the rate of local-regional control was 75%, and the rate of distant control was 76%. Radiation reactions in the skin and febrile neutropenia were significant clinical acute toxicities. Because of the heterogeneity of the population and the nonstandardized surgery, it is difficult to infer a clear treatment benefit from the chemotherapy.[Level of evidence C1]
In a subsequent report, the same investigators evaluated a subset of these protocol patients (n = 40, after excluding patients with unknown primaries) and compared them with 61 historical controls who received no chemotherapy, were treated at the same institutions, were diagnosed before 1997, and had no routine imaging staging studies. Radiation therapy was given to 50 patients. There was no significant survival benefit seen for chemotherapy patients.
In a subsequent pilot clinical trial of 18 patients from 2004 to 2006, the same investigators attempted to reduce the skin and hematological toxicity seen in Study 96-07. Carboplatin (AUC = 2) was administered weekly during radiation therapy beginning on day 1 for a maximum of five doses, followed by three cycles of carboplatin (AUC 4.5, and IV etoposide 80 mg/m2 on days 1–3 beginning 3 weeks after radiation and repeated every 3 weeks for three cycles). The radiation therapy was similar to that in the earlier trial. Results suggested a decrease in hematological and skin toxicity.
Use of chemotherapy has also been reported in selected patients with locally advanced and metastatic disease. In one retrospective study of 107 patients, 57% of patients with metastatic disease and 69% with locally advanced disease responded to initial chemotherapy. Median OS was 9 months for patients with metastatic disease and 24 months for patients with locally advanced disease. At 3 years, the OS rate was projected to be 17% for those with metastatic disease and 35% for those with locally advanced disease. Toxicity was significant, however, and without clear benefit, particularly in older patients.[Level of evidence C2]
Follow-up
The most appropriate follow-up techniques and frequency for patients treated for MCC have not been prospectively studied. Because of the propensity for local and regional recurrence, clinicians should perform at least a thorough physical examination of the site of initial disease and the regional lymph nodes. Imaging studies may be ordered to evaluate signs and symptoms of concern, or they may be performed to identify distant metastases early; but, there are no data suggesting that early detection and treatment of new distant metastases results in improved survival.
In one series of 237 patients presenting with local or regional disease, the median time-to-recurrence was 9 months (range, 2–70 months). Ninety-one percent of recurrences occurred within 2 years of diagnosis. It has been suggested that the intensity of follow-up can be gradually diminished after 2 to 3 years as the majority of recurrences are likely to have already occurred.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Busse PM, Clark JR, Muse VV, et al.: Case records of the Massachusetts General Hospital. Case 19-2008. A 63-year-old HIV-positive man with cutaneous Merkel-cell carcinoma. N Engl J Med 358 (25): 2717-23, 2008.
- Medina-Franco H, Urist MM, Fiveash J, et al.: Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol 8 (3): 204-8, 2001.
- Nghiem P, James N: Merkel cell carcinoma. In: Wolff K, Goldsmith LA, Katz SI, et al., eds.: Fitzpatrick's Dermatology in General Medicine. 7th ed. McGraw-Hill , 2008, pp 1087-94.
- Allen PJ, Bowne WB, Jaques DP, et al.: Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol 23 (10): 2300-9, 2005.
- Goessling W, McKee PH, Mayer RJ: Merkel cell carcinoma. J Clin Oncol 20 (2): 588-98, 2002.
- Senchenkov A, Barnes SA, Moran SL: Predictors of survival and recurrence in the surgical treatment of merkel cell carcinoma of the extremities. J Surg Oncol 95 (3): 229-34, 2007.
- Nghiem P, McKee PH, Haynes HA: Merkel cell (cutaneous neuroendocrine) carcinoma. In: Sober AJ, Haluska FG, eds.: Skin Cancer. BC Decker Inc., 2001, pp 127-141.
- Boyer JD, Zitelli JA, Brodland DG, et al.: Local control of primary Merkel cell carcinoma: review of 45 cases treated with Mohs micrographic surgery with and without adjuvant radiation. J Am Acad Dermatol 47 (6): 885-92, 2002.
- Wilson LD, Gruber SB: Merkel cell carcinoma and the controversial role of adjuvant radiation therapy: clinical choices in the absence of statistical evidence. J Am Acad Dermatol 50 (3): 435-7; discussion 437-8, 2004.
- Gollard R, Weber R, Kosty MP, et al.: Merkel cell carcinoma: review of 22 cases with surgical, pathologic, and therapeutic considerations. Cancer 88 (8): 1842-51, 2000.
- Eng TY, Boersma MG, Fuller CD, et al.: A comprehensive review of the treatment of Merkel cell carcinoma. Am J Clin Oncol 30 (6): 624-36, 2007.
- Gupta SG, Wang LC, Peñas PF, et al.: Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: The Dana-Farber experience and meta-analysis of the literature. Arch Dermatol 142 (6): 685-90, 2006.
- Stokes JB, Graw KS, Dengel LT, et al.: Patients with Merkel cell carcinoma tumors < or = 1.0 cm in diameter are unlikely to harbor regional lymph node metastasis. J Clin Oncol 27 (23): 3772-7, 2009.
- Haag ML, Glass LF, Fenske NA: Merkel cell carcinoma. Diagnosis and treatment. Dermatol Surg 21 (8): 669-83, 1995.
- Ratner D, Nelson BR, Brown MD, et al.: Merkel cell carcinoma. J Am Acad Dermatol 29 (2 Pt 1): 143-56, 1993.
- Yiengpruksawan A, Coit DG, Thaler HT, et al.: Merkel cell carcinoma. Prognosis and management. Arch Surg 126 (12): 1514-9, 1991.
- Messina JL, Reintgen DS, Cruse CW, et al.: Selective lymphadenectomy in patients with Merkel cell (cutaneous neuroendocrine) carcinoma. Ann Surg Oncol 4 (5): 389-95, 1997 Jul-Aug.
- Hill AD, Brady MS, Coit DG: Intraoperative lymphatic mapping and sentinel lymph node biopsy for Merkel cell carcinoma. Br J Surg 86 (4): 518-21, 1999.
- Wasserberg N, Schachter J, Fenig E, et al.: Applicability of the sentinel node technique to Merkel cell carcinoma. Dermatol Surg 26 (2): 138-41, 2000.
- Rodrigues LK, Leong SP, Kashani-Sabet M, et al.: Early experience with sentinel lymph node mapping for Merkel cell carcinoma. J Am Acad Dermatol 45 (2): 303-8, 2001.
- Mehrany K, Otley CC, Weenig RH, et al.: A meta-analysis of the prognostic significance of sentinel lymph node status in Merkel cell carcinoma. Dermatol Surg 28 (2): 113-7; discussion 117, 2002.
- Schwartz JL, Griffith KA, Lowe L, et al.: Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol 29 (8): 1036-41, 2011.
- Fields RC, Busam KJ, Chou JF, et al.: Recurrence and survival in patients undergoing sentinel lymph node biopsy for merkel cell carcinoma: analysis of 153 patients from a single institution. Ann Surg Oncol 18 (9): 2529-37, 2011.
- Maza S, Trefzer U, Hofmann M, et al.: Impact of sentinel lymph node biopsy in patients with Merkel cell carcinoma: results of a prospective study and review of the literature. Eur J Nucl Med Mol Imaging 33 (4): 433-40, 2006.
- Goepfert H, Remmler D, Silva E, et al.: Merkel cell carcinoma (endocrine carcinoma of the skin) of the head and neck. Arch Otolaryngol 110 (11): 707-12, 1984.
- Lewis KG, Weinstock MA, Weaver AL, et al.: Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol 142 (6): 693-700, 2006.
- Veness MJ, Perera L, McCourt J, et al.: Merkel cell carcinoma: improved outcome with adjuvant radiotherapy. ANZ J Surg 75 (5): 275-81, 2005.
- Jabbour J, Cumming R, Scolyer RA, et al.: Merkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage disease--results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann Surg Oncol 14 (6): 1943-52, 2007.
- Veness M, Foote M, Gebski V, et al.: The role of radiotherapy alone in patients with merkel cell carcinoma: reporting the Australian experience of 43 patients. Int J Radiat Oncol Biol Phys 78 (3): 703-9, 2010.
- Meeuwissen JA, Bourne RG, Kearsley JH: The importance of postoperative radiation therapy in the treatment of Merkel cell carcinoma. Int J Radiat Oncol Biol Phys 31 (2): 325-31, 1995.
- Marks ME, Kim RY, Salter MM: Radiotherapy as an adjunct in the management of Merkel cell carcinoma. Cancer 65 (1): 60-4, 1990.
- Mojica P, Smith D, Ellenhorn JD: Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J Clin Oncol 25 (9): 1043-7, 2007.
- Housman DM, Decker RH, Wilson LD: Regarding adjuvant radiation therapy in merkel cell carcinoma: selection bias and its affect on overall survival. J Clin Oncol 25 (28): 4503-4; author reply 4504-5, 2007.
- Garneski KM, Nghiem P: Merkel cell carcinoma adjuvant therapy: current data support radiation but not chemotherapy. J Am Acad Dermatol 57 (1): 166-9, 2007.
- Foote M, Harvey J, Porceddu S, et al.: Effect of radiotherapy dose and volume on relapse in Merkel cell cancer of the skin. Int J Radiat Oncol Biol Phys 77 (3): 677-84, 2010.
- Fang LC, Lemos B, Douglas J, et al.: Radiation monotherapy as regional treatment for lymph node-positive Merkel cell carcinoma. Cancer 116 (7): 1783-90, 2010.
- Becker JC, Lorenz E, Ugurel S, et al.: Evaluation of real-world treatment outcomes in patients with distant metastatic Merkel cell carcinoma following second-line chemotherapy in Europe. Oncotarget 8 (45): 79731-79741, 2017.
- Kaufman HL, Russell JS, Hamid O, et al.: Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer 6 (1): 7, 2018.
- Cowey CL, Mahnke L, Espirito J, et al.: Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol 13 (19): 1699-1710, 2017.
- Nghiem PT, Bhatia S, Lipson EJ, et al.: PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med 374 (26): 2542-52, 2016.
- Topalian SL, Bhatia S, Hollebecque A, et al.: Abstract CT074: Non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus-associated tumors (CheckMate 358): efficacy and safety in Merkel cell carcinoma (MCC). [Abstract] Cancer Res 77 (13 Suppl): A-CT074, 2017.
- Tai PT, Yu E, Winquist E, et al.: Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol 18 (12): 2493-9, 2000.
- Henness S, Vereecken P: Management of Merkel tumours: an evidence-based review. Curr Opin Oncol 20 (3): 280-6, 2008.
- Poulsen M, Rischin D, Walpole E, et al.: High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group Study--TROG 96:07. J Clin Oncol 21 (23): 4371-6, 2003.
- Poulsen MG, Rischin D, Porter I, et al.: Does chemotherapy improve survival in high-risk stage I and II Merkel cell carcinoma of the skin? Int J Radiat Oncol Biol Phys 64 (1): 114-9, 2006.
- Poulsen M, Walpole E, Harvey J, et al.: Weekly carboplatin reduces toxicity during synchronous chemoradiotherapy for Merkel cell carcinoma of skin. Int J Radiat Oncol Biol Phys 72 (4): 1070-4, 2008.
- Voog E, Biron P, Martin JP, et al.: Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma. Cancer 85 (12): 2589-95, 1999.
Treatment of Stage I and II Merkel Cell Carcinoma
Stage I and II Merkel cell carcinoma (MCC) include patients with local disease only.
Excision with 1 cm to 2 cm margins and radiation therapy are the mainstays of management for primary MCC tumors. Adjuvant radiation therapy to the primary tumor site is often recommended; however, the morbidity of radiation may be avoided and low local recurrence rates maintained, as shown in a subset of patients with small low-risk lesions (i.e., tumors <2 cm without other adverse prognostic factors).
Because of the risk of occult nodal disease, sentinel lymph node (SLN) biopsy is recommended for patients without clinically detectable metastatic disease. Any size of metastatic deposit is currently considered positive with regard to regional lymph node (N) staging; therefore, immunohistochemistry is routinely used to improve detection of micrometastases in SLN.
Treatment Options for Stage I and II Merkel Cell Carcinoma
Treatment options for stage I and stage II MCC include the following:
- Margin-negative local excision, attempting to maintain function.
- Surgical nodal evaluation, typically by SLN procedure initially, may be considered for patients with significant risk of nodal disease. Completion of the nodal dissection may be considered if positive lymph nodes are found, which would upstage the patient's cancer to stage III.
- Local radiation therapy may be considered if there is concern about the primary tumor excision margins. Regional radiation therapy may be considered if the nodal staging procedure is incomplete or omitted. For sites where the location of primary regional lymph nodes may be uncertain (e.g., mid-back), regional-node field selection is problematic.
- Enrollment in clinical trials is encouraged.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Frohm ML, Griffith KA, Harms KL, et al.: Recurrence and Survival in Patients With Merkel Cell Carcinoma Undergoing Surgery Without Adjuvant Radiation Therapy to the Primary Site. JAMA Dermatol 152 (9): 1001-7, 2016.
- Cassler NM, Merrill D, Bichakjian CK, et al.: Merkel Cell Carcinoma Therapeutic Update. Curr Treat Options Oncol 17 (7): 36, 2016.
- Harms PW: Update on Merkel Cell Carcinoma. Clin Lab Med 37 (3): 485-501, 2017.
- Su LD, Lowe L, Bradford CR, et al.: Immunostaining for cytokeratin 20 improves detection of micrometastatic Merkel cell carcinoma in sentinel lymph nodes. J Am Acad Dermatol 46 (5): 661-6, 2002.
Treatment of Stage III Merkel Cell Carcinoma
Stage III Merkel cell carcinoma (MCC) includes patients with nodal disease.
Treatment Options for Stage III Merkel Cell Carcinoma
Treatment options for stage III MCC include the following:
- Margin-negative local excision, attempting to maintain function.
- Sentinel lymph node procedure, possibly followed by more definitive regional node surgery if positive node(s) are found.
- Local and regional lymph node radiation, especially if there is concern about the adequacy of the primary tumor excision margin or the risk of local-regional recurrence following the nodal surgery (e.g., multiple primary nodes, extracapsular extension, lymphovascular invasion, and evidence of in-transit metastases).
- Systemic chemotherapy may be administered to patients with the highest risk of recurrence with the understanding that existing data have not proven a clinical survival benefit (under clinical evaluation).
- Enrollment in clinical trials is encouraged.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
stage IV Merkel cell carcinomaTreatment of Stage IV Merkel Cell Carcinoma
Stage IV Merkel cell carcinoma (MCC) includes patients with distant metastases.
Chemotherapy may be considered for patients with stage IV disease who have a good performance status. Although responses have been seen with various regimens, evidence is lacking that chemotherapy results in permanent disease control or long-term survival.
In stage IV patients for whom chemotherapy is not considered an appropriate option, surgery and/or radiation therapy may be considered for local or regional palliation.
Avelumab, an anti-programmed death ligand-1 antibody, has been approved by the U.S. Food and Drug Administration as therapy for metastatic MCC. Other immunotherapies for MCC are under clinical evaluation. The success of immune-based therapies represents a milestone in the management of advanced MCC. However, not all patients respond to immune-based therapies. Furthermore, patients requiring immunosuppression in the setting of solid organ transplants or those with autoimmune disease may not be optimal candidates for immune-based therapy.
Treatment Options for Stage IV Merkel Cell Carcinoma
Treatment options for stage IV MCC include the following:
- Palliation with chemotherapy and/or surgery and/or radiation therapy as clinically appropriate.
- Enrollment in clinical trials is strongly encouraged.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
recurrent Merkel cell carcinomaTreatment of Recurrent Merkel Cell Carcinoma
Merkel cell carcinoma is a rare tumor. There are no clinical trials reported for patients with recurrent disease exclusively. Recommendations and outcomes of various treatments for these patients are included in many large case series [Level of evidence C2] and one phase II clinical trial.[Level of evidence C1] Treatments are usually individualized based on patient preference and the specifics of each case, and there are no standard options. Consideration should be given to enrollment in clinical trials.
Treatment Options for Recurrent Merkel Cell Carcinoma
Local recurrence
Treatment options for patients with local recurrence include wider local surgery if possible, followed by radiation therapy, if not previously given.
Regional lymph node dissection (RLND) can also be considered if regional draining nodes have not been previously removed.
Because of the poor prognosis after recurrence, consideration can also be given to systemic chemotherapy, although there is no evidence that it improves survival.
Nodal recurrence
Treatment options for patients with only regional nodal recurrence include RLND and adjuvant radiation therapy if the regional draining nodes have not been previously treated. Because of the poor prognosis after recurrence, consideration can also be given to systemic chemotherapy, although there is no evidence that it improves survival.
Distant recurrence
For patients with distant recurrence only, chemotherapy is an option for patients who have good performance status.[Level of evidence C2] Although responses with chemotherapy have been reported in selected patients with locally advanced and metastatic disease, toxicity has been significant and without clear benefit, particularly in older patients. When appropriate, radiation therapy and/or surgery may be offered as palliation to sites of recurrence, particularly if chemotherapy is not considered an option.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Goessling W, McKee PH, Mayer RJ: Merkel cell carcinoma. J Clin Oncol 20 (2): 588-98, 2002.
- Henness S, Vereecken P: Management of Merkel tumours: an evidence-based review. Curr Opin Oncol 20 (3): 280-6, 2008.
- Voog E, Biron P, Martin JP, et al.: Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma. Cancer 85 (12): 2589-95, 1999.
- Poulsen M, Rischin D, Walpole E, et al.: High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group Study--TROG 96:07. J Clin Oncol 21 (23): 4371-6, 2003.
- Eng TY, Boersma MG, Fuller CD, et al.: A comprehensive review of the treatment of Merkel cell carcinoma. Am J Clin Oncol 30 (6): 624-36, 2007.
- Tai PT, Yu E, Winquist E, et al.: Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol 18 (12): 2493-9, 2000.
Latest Updates to This Summary (10/28/2022)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of merkel cell carcinoma. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewer for Merkel Cell Carcinoma Treatment is:
- Russell S. Berman, MD (New York University School of Medicine)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Merkel Cell Carcinoma Treatment. Bethesda, MD: National Cancer Institute. Updated
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.