Chemotherapy Primer: Why? What? and How?
A special note to the reader: all the chemotherapy drugs discussed herein can be found on OncoLink Rx.
History of chemotherapy
Chemotherapy, or the use of chemical agents to destroy cancer cells, is a mainstay in the treatment of malignancies. The possible role in treating illness was discovered when the bone marrow suppressive effect of nitrogen mustard was noted in the early 1900's. Since that time, the search for drugs with anticancer activity has continued, and the goal of treatment with chemotherapy has evolved from relief of symptoms to cancer cure. A major advantage of chemotherapy is its ability to treat widespread or metastatic cancer, whereas surgery and radiation therapy are limited to treating cancers that are confined to specific areas.
How do chemotherapy drugs work?
The goal of all chemotherapy drugs is to kill the cancerous cells, while using a dose that causes the least harm the body's healthy cells. To achieve this goal, scientists tried to identify characteristics that are unique to cancer cells and are not found on normal tissue. A distinct cancer cell trait could serve as a potential target for a chemotherapy drug and thereby spare normal tissues. One feature that is seen in most cancer cells is that they grow at a rate faster than normal cells. Therefore, targeting some aspect of the cell growth cycle seems reasonable. Fast-growing cells would be affected the most and slow-growing cells would be least disturbed. In fact, that is the basis for many chemotherapy agents. This is apparent when considering the side effect profiles of most chemotherapy drugs. Hair follicles, skin, and the cells that line the gastrointestinal tract are some of the fastest growing cells in the human body, and therefore are most sensitive to the effects of chemotherapy. It is for this reason that patients may experience hair loss, rashes, and diarrhea, respectively.
The human body processes and excretes all drugs through either the liver or the kidneys. Therefore, when a patient has kidney or liver damage, giving chemotherapy becomes precarious. Administering the recommended amount of drug may prove to be too toxic in a patient unable to metabolize and excrete it. The pharmacokinetics (how the body handles a drug) for cancer patients are very complex, and chemotherapy pharmacology is a subspecialty on its own. Unfortunately, kidney and liver damage often result due to cancer invasion, possibly limiting the patient's chemotherapy options.
Pharmacokinetics is further complicated in cancer patients, as they are often taking multiple medications, some of which have overlapping metabolic pathways and side effect profiles. An example of this difficult situation is in the brain cancer patient. Because brain tumors often present with seizures, many of these patients take anti-seizure medications. Anti-seizure medications are metabolized by the liver and affect the metabolism of many chemotherapy drugs. Dose adjustments are an absolute necessity to avoid toxicities or sub-therapeutic dosing (doses that are too low) in these patients.
The cell cycle
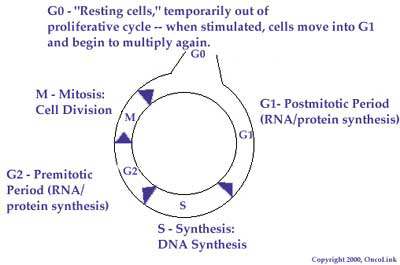
An understanding of the normal cell cycle and the behavior of malignant or cancerous cells can help one better understand how chemotherapy works to destroy cancer cells. The cell cycle is the sequence of steps a cell goes through in order to copy its genetic material and divide into two cells. The cell cycle is divided into four phases: the G 1, S, G 2, and M phases. A chemotherapy agent may work in only one phase of the cycle (called cell-cycle specific) or be active in all phases (cell-cycle nonspecific).
The G 1 phase is the phase most active in protein synthesis. The cellular DNA at this phase is tightly coiled and is not actively being transcribed (copied). Few chemotherapy agents are active at this phase of the cell cycle. By contrast, the S phase is the synthetic phase of the cell cycle. DNA replication is most active in this phase and many chemotherapy agents work in this phase. G 2 represents a time when mostly RNA (and some protein) is actively produced. Mitosis, or actual cell division, occurs during the M phase. There are two major classes of chemotherapy drugs that are most active during this phase of the cell cycle. By knowing the phase an agent works in, we are able to combine agents that work in different phases to achieve the most tumor killing.
Chemotherapeutic Agents
Most chemotherapy agents kill cancer cells by affecting DNA synthesis or function, a process that occurs through the cell cycle. Each drug varies in the way this occurs within the cell cycle.
The major categories of chemotherapy agents are alkylating agents, antimetabolites, anthracyclines, plant alkaloids, antitumor antibiotics, taxanes, and monoclonal antibodies. Let's review the characteristics of these groups.
Alkylating agents
Alkylating agents are the oldest class of anticancer drugs. Almost all of these drugs are active or latent nitrogen mustards. Nitrogen mustards are various poisonous compounds originally developed for military use. Alkylating agents all share a common mechanism of action, but differ in their clinical activity. They work by attacking the negatively charged sites on the DNA (oxygen, nitrogen, phosphorous and sulfur atoms). By binding to the DNA, steps (replication, transcription, and base pairing) leading to duplication of the cell's genetic material are significantly altered. In addition, alkylation of DNA leads to DNA strand breaks and DNA strand cross-linking. By altering DNA in this manner, cellular activity is stopped and the cell dies. Chemotherapy drugs in this class are active in every phase of the cell cycle. As a result, this class of anticancer drugs is very powerful and is used in many types of cancer, including both solid tumors and leukemias.
In general, prolonged use of these drugs will lead decreased sperm production, cessation of menstruation, and possibly cause permanent infertility. This class of chemotherapeutics should never be used in the first trimester of pregnancy as they have been shown to increase fetal malformations. Use in the second or third trimester does not seem to carry the same risk. All alkylating agents can cause secondary cancers although not all agents are equal in their carcinogenic potential. The most common secondary cancer is a type of leukemia (AML, or Acute Myeloid Leukemia) that can occur years after therapy with an alkylating agent.
Natural metal derivatives, called platinums, are a group of "alkylating-like" agents. Although, structurally, they do not contain an alkyl group, they are similar to alkylating agents because they cross-link DNA, resulting in impairment of DNA synthesis, transcription and function. The platinum compounds can act in any cell cycle. Cisplatin is used most often in lung, head and neck, cervical, and testicular cancer. The most significant toxicity of cisplatin is kidney damage. A second-generation platinum, called carboplatin, has fewer kidney side effects, and at times may be an appropriate substitute for regiments containing cisplatin. Oxaliplatin is a third-generation platinum that is active in colon cancer and has no renal (kidney) toxicities, however, it can cause severe neuropathies.
Other common alkylating agents include: Cyclophosphamide, Ifosfamide, Melphalan, Chlorambucil, BCNU, CCNU, Dacarbazine, Procarbazine, Busulfan, and Thiotepa.
Antimetabolites
In 1948, Dr. Sidney Farber showed that a folic acid analog could induce remission in childhood leukemia. Approximately 10 out of the 16 patients treated demonstrated evidence of hematologic improvement. This experience provided the foundation for scientists to synthesize a number of other agents that either target naturally occurring compounds or inhibit key enzymatic reactions in their biochemical pathways. In general, all antimetabolites interfere with normal metabolic pathways. Because antimetabolites are mistaken by the cell for normal metabolites, they prevent the normal metabolites from becoming incorporated into DNA, stopping normal development and division. The most widely used antifolate in cancer therapy, with activity against leukemia, lymphoma, breast cancer, head and neck cancer, sarcomas, colon cancer, bladder cancer and choriocarcinomas, is Methotrexate. Methotrexate inhibits a crucial enzyme required for DNA synthesis and therefore exerts its effect on the S phase of the cell cycle.
5-Fluorouracil (5-FU), another widely used antimetabolite, prevents DNA synthesis by interfering with the nucleotide (DNA components) production. It, too, has a wide range of activity including colon cancer, breast cancer, head and neck cancer, pancreatic cancer, gastric cancer, anal cancer, esophageal cancer and hepatomas (primary liver tumor). A topical form of 5-FU is sometimes used to treat skin cancer. A unique and interesting aspect of this drug is its toxicity profile. 5-Fluorouracil is metabolized by a naturally occurring enzyme in the body called dihydropyrimidine dehydrogenase, or DPD. There is a small population of people who are deficient of this particular enzyme. Lacking DPD does not interfere with normal body function and thus people are not aware that they are lacking it. However, when these patients are given this chemotherapy drug, they are unable to metabolize it and therefore get acute and severe toxicities (side effects). The most often seen toxicities include bone marrow suppression, severe GI toxicities, and neurotoxicities which may include seizures and even coma. It is important for the oncologist to recognize this early and give the patient Thymidine or Vistonuridine as an antidote. A drug called Capecitabine is an oral type of 5-Fluorouracil compound that has similar side effect potentials.
Other antimetabolites that inhibit DNA synthesis and DNA repair include: Cytarabine, Gemcitabine ( Gemzar ®), 6 mercaptopurine, Thioguanine, Fludarabine, and Cladribine.
Anthracyclines
Many of the currently effective anti-cancer drugs are developed from natural sources. The drug daunorubicin was isolated from Streptomyces, a soil-dwelling fungus. Doxorubicin, another anthracycline drug, was isolated from a mutated strain of the same fungus. Both of these drugs have a similar mechanism of action, but the latter is more effective in the treatment of solid tumors. This class of chemotherapeutics works by the formation of free oxygen radicals. These radicals result in DNA strand breaks and subsequent inhibition of DNA synthesis and function. Anthracyclines also inhibit the enzyme topoisomerase by forming a complex with the enzyme and DNA. Topoisomerases are a class of enzymes that serve to unwind the DNA double strand helix to allow for DNA repair, replication and transcription. This class of chemotherapeutics is also not cell cycle specific. The most important side effect of this group of drugs is cardiac toxicity. The same free radicals that serve to damage the DNA of the cancer cell may damage the cells of the heart muscle. Oncologists monitor heart function very carefully when patients are on these medications. Other commonly used anthracyclines include Idarubicin, Epirubicin, and Mitoxantrone.
Antitumor Antibiotics
Another chemotherapy isolated form the fungus Streptomyces verticullus is Bleomycin. Its mechanism of action is similar to that of the anthracyclines, in that free oxygen radicals are formed that result in DNA breaks leading to cancer cell death. This drug is rarely used by itself rather in conjunction with other chemotherapies. Bleomycin is an active agent in regimens for testicular cancer and Hodgkin's lymphoma. The most concerning side effect of this drug is lung toxicities due to oxygen free radical formation.
Plant Alkaloids
Plant alkaloids are a group of chemotherapy agents derived from plant materials. They are broken down into four groups: topoisomerase inhibitors, epipodophyllotoxins, taxanes and vinca alkaloids. Plant alkaloids are cell-cycle specific, but the cycle affected varies from drug to drug.
Camptothecan analogs (also called Topoisomerase I inhibitors) act by forming a complex with Topoisomerase and DNA resulting in the inhibition and function of the Topoisomerase enzyme. The presence of Topoisomerase is required for on-going DNA synthesis. These drugs are used in many solid and liquid tumors. Unlike other classes of chemotherapy, the side effects of this class of drugs vary from drug to drug.
Camptothecins include both irinotecan and topotecan. The parent compound of these agents, first identified in the late 1950's, is a naturally occurring alkaloid found in the bark and wood of the Chinese tree Camptotheca accuminata, also called the "Happy Tree."
Etoposide and Teniposide are epipodophyllotoxin chemotherapy agents (also called topoisomerase II inhibitors) that work by similar mechanisms. They are isolated from the May Apple plant and work in the late S and G 2 phases.
The leaves of a periwinkle plant, Vinca rosea, were used by natives of Madagascar to make tea that reportedly improved diabetes. Although it did not affect blood sugar levels in research studies, it was found that the extract killed cells found in leukemias. Isolation and chemical characterization lead to the currently used chemotherapy drugs: vincristine, vinblastine, and vinorelbine. These chemotherapeutics bind to the tubulin and lead to the disruption of the mitotic spindle apparatus. The disruption of mitosis implies that these drugs are active specifically during the M phase of the cell cycle. They have a wide application to many different malignancies and cause neurotoxicity as the most prominent and dose limiting side effect.
Taxanes
Another class of chemotherapeutics that are specific for the M phase of the cell cycle is the Taxanes. The taxanes include paclitaxel and docetaxel. They bind with high affinity to the microtubules and inhibit their normal function. This class of drugs has a broad range of clinical activity including breast cancer, lung cancer, head and neck cancer, ovarian cancer, bladder cancer, esophageal cancer, gastric cancer and prostate cancer. The most common side effect of these drugs is the lowering of the blood counts. These compounds were first isolated from the bark of the Pacific yew tree Taxus brevifolia in 1963. It was not until 1971 that paclitaxel was identified as the active component and 1993 before it was available for use.
Monoclonal Antibodies
This a relatively new classification of drugs which, unlike the types of chemotherapy previously mentioned, depends on an understanding of molecular events responsible for the development of the cancer being treated. Monoclonal antibodies bind only to cancer cell-specific antigens and induce an immunological response against the target cancer cell. Trastuzumab (brand name Herceptin) is one of the first targeted agents to be approved for cancer therapy. Trastuzumab is an antibody to the cell-surface receptor of the epidermal growth factor receptor (EGFR) family. Certain breast cancers are known to have an increased amount of EGFR, and thus are amenable to blockage of the receptor. Targeted agents used to treat other types of cancer include Imitinab, Cetuximab and Bevacizumab.
Miscellaneous Chemotherapy Agents
Certain types of chemotherapies are orphan drugs, meaning there are no others like them. Examples of miscellaneous agents include: L-asparaginase, hydroxyurea, thalidomide and dactinomycin.
Chemotherapy Administration
The most common routes of administration for chemotherapy are by mouth, through a vein, and into a muscle. More recently, other methods have been used to increase the local concentration of chemotherapy at the tumor site. Chemotherapy can be administered directly into a specific cavity (intracavitary), the abdomen (intraperitoneal), the lung (intrapleural), the central nervous system (intra-thecal), or applied directly to the skin (topical).
Because many chemotherapeutic agents also affect healthy cells and organs, the patient's laboratory data should be checked before chemotherapy administration, including white blood cell count, hemoglobin/hematocrit, platelet count, renal function tests, and liver function tests. In addition, assessment for organ specific drug effects will be performed on a periodic basis. Abnormalities in any of these values may require dose adjustments or the delay of therapy. Additionally, pretreatment actions, such as increased intravenous fluids or administration of anti-nausea medicines may be needed to decrease side effects.
Strategies of Chemotherapy Administration
Combination Chemotherapy combines agents that differ in both the way they act and their side effects. This is done to achieve maximum tumor effect with minimal side effects. Because tumor cells have different biological characteristics (heterogeneity), combining drugs may effectively eliminate cancer cells' ability to gain resistance to a single agent. By giving multiple "cycles" of chemotherapy, over a period of months, at specific time intervals (i.e. every 3 weeks, weekly), chemotherapy can destroy tumor cells while allowing the patient's normal cells time to heal.
Primarychemotherapy (treatment with chemotherapy only) is given when chemotherapy, on its own, is expected to control or cure the cancer; it can be given for acute treatment or long-term control. Primary chemotherapy is the treatment of choice for some leukemias and lymphomas.
Adjuvant Chemotherapy (chemotherapy given after surgery) is used in this manner to decrease the risk of the cancer coming back. This is done even when no clear evidence of cancer can be found, but certain factors (e.g. metastasis to the lymph nodes, large tumor size) predict an increased risk of cancer recurrence.
Neoadjuvant Chemotherapy (chemotherapy given before surgery) is used in this manner to shrink a tumor before surgery, which may allow the surgeon to perform a smaller surgery and/or remove all visible tumor.
Combined Modality Chemotherapy is the practice of using chemotherapy in combination with other treatment modalities, such as radiation or surgery. Therapies are combined to obtain a greater response rate than could be achieved with a single treatment modality. Today, using more than one treatment modality is common for most cancers.
Hormonal Manipulation does not directly kill cells and, therefore, is not curative. Their purpose is to prevent cell division and further growth of hormone-dependent tumors. Their use is frequently reserved for the management of patients with locally advanced or metastatic cancer. Visit the hormonal therapy section for more information.
Investigational Therapy
The identification and development of effective new anticancer drugs is an ongoing process. Following rigorous testing in laboratory animals and experimental model systems, chemotherapy agents with demonstrated antitumor activity are evaluated in clinical trials. In phase I trials, the initial phase of clinical investigation, a new treatment is evaluated in cancer patients for the first time. The purpose of these studies is to determine the associated side effects, the highest dose safely tolerated, and the optimal schedule or mode of delivery of a new therapy. Phase II trials test a new therapy (using the dose, method of administration, and schedule defined in Phase I) in patients with a variety of tumors to determine whether there is identifiable antitumor activity. In Phase III trials, new therapies that exhibited activity in Phase II are compared in larger numbers of patients to the standard or best available therapy for each type of tumor tested.
Participation in a clinical trial is one treatment option, which may be offered to patients at some point during therapy. The continuing progress of cancer treatment depends upon the participation of adequate numbers of patients in such studies.